Follow On Submissions (Amendment, Annual Continuation, and Triennial Renewal)
If you need to make changes to an approved protocol or review it, you can create the following submissions. If you are creating a follow-on submission to a protocol that was originally approved in Coeus and has not had a follow-on submission in PERA, see additional guide here.
- Continuing Review: A review of an approved protocol that is conducted at appropriate intervals between triennial reviews, usually annually.
- Triennial Review: A review of an approved protocol that is conducted every three years.
- Amendment: Created to submit a change to an approved protocol.
![]() NOTE
NOTE
For continuing (annual) reviews, make sure you review all protocol information (e.g., team members, funding sources, activities, and approved and used animal numbers) and submit any needed amendments immediately. If an amendment is already in review, wait until the first amendment is processed. Only one follow-on submission may be created at a time for a protocol.
Migrated Protocols
If you are working on a protocol that was migrated from Coeus to PERA, there are some additional steps you will need to follow. Select the button below to learn more.
Content

Create a Follow-On Submission
- From the Dashboard, click on the IACUC tab.
- Click the All Submissions tab.
- Select the name of the approved protocol.

4. Click the type of follow-on submission you want to create (i.e. Continuing Review, Triennial Review, or Amendment).

5. Complete the pages. See below for more details on each page. Click Continue to move on to the next page.

6. When done, click Exit and save changes or click Finish on the final page.
Create Continuing Review
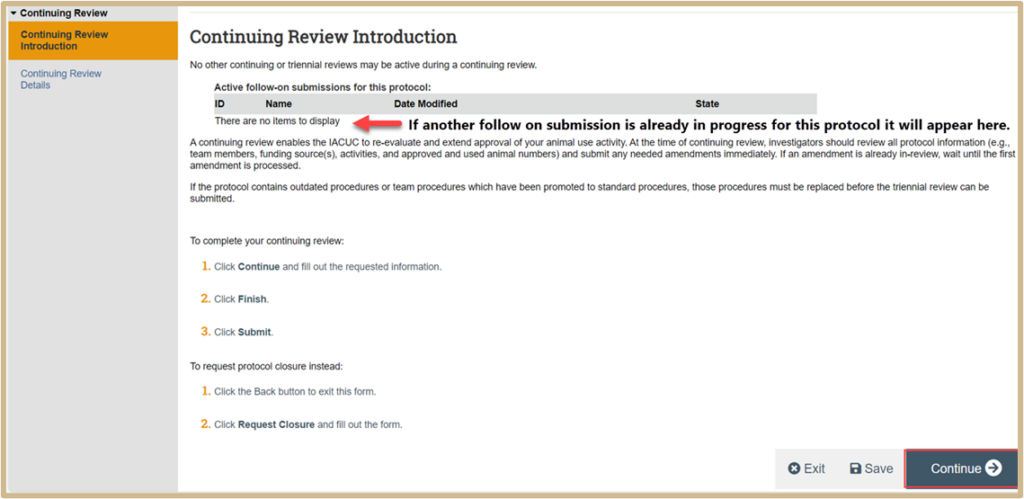
Read through the Continuing Review Introduction page. If you would like to proceed with creating a Continuing Review, click Continue.


 Only if Yes is selected on #2.
Only if Yes is selected on #2.

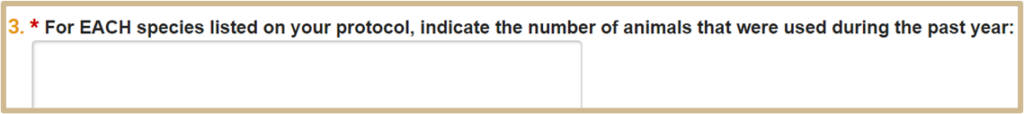

 Only if Yes is selected on #4.
Only if Yes is selected on #4.


Click Finish when done.
Create Triennial Review

Read through the Triennial Review Introduction page. If you would like to proceed with creating a Triennial Review, click Continue.
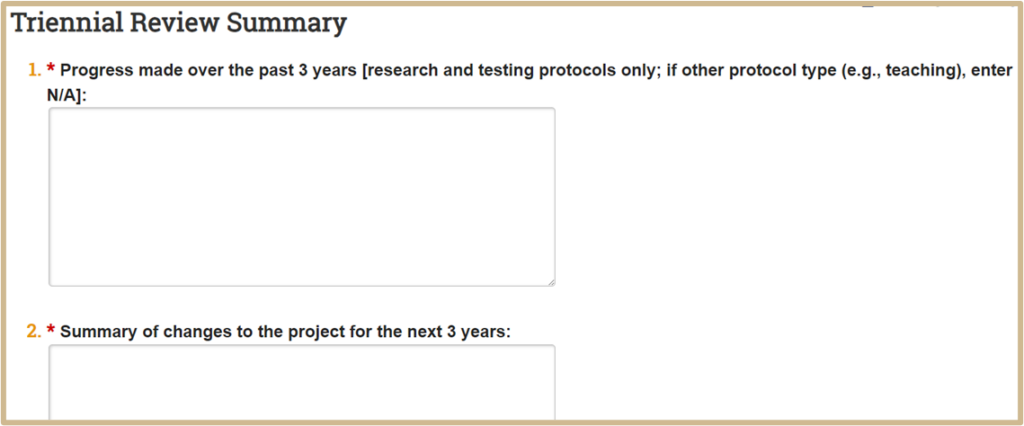

![]() Examples of unanticipated results are:
Examples of unanticipated results are:
- The number of animals required was higher than initially anticipated
- Actual experiment results were different than expected
You could also list a summary of amendments submitted during the current review period and reasons for them.

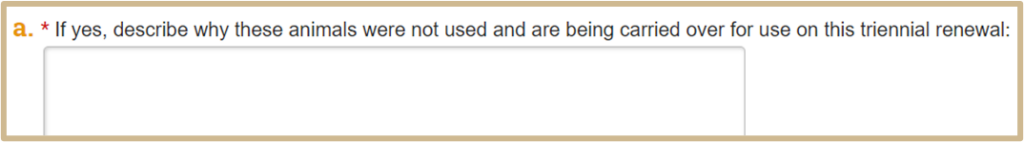
 Only if Yes is selected on #4.
Only if Yes is selected on #4.


 Only if Yes is selected on #5.
Only if Yes is selected on #5.
Click Continue to next page, Triennial Review Details.
This will take you to the approved protocol. Go through each of the pages to review each section and update as needed.
Click Finish when done.
Create Amendment

Click Continue to proceed to Amendment Details.
This will take you to the protocol application where you can make your changes. Go through each of the pages to review and change the sections that you want to amend.
Click Finish when done.
Submit Follow-On Submission for Review
Before submitting your Follow-On Submission, you can use the Validate button to confirm that all required questions have been answered. This will direct you to the questions that still require an answer to submit.

Once all required questions have been answered, you can exit the protocol and will be on the submission workspace.

From the follow-on submission’s workspace, click Submit.

Content Updated:


