 PERA IRB Administrative Staff Procedure Guide
PERA IRB Administrative Staff Procedure Guide
12. Report New Information (RNI)

12. Report New Information (RNI)
Anyone that has access to the system will be able to create an RNI.
There are a few different ways that you can create a new RNI within the system.
Option #1
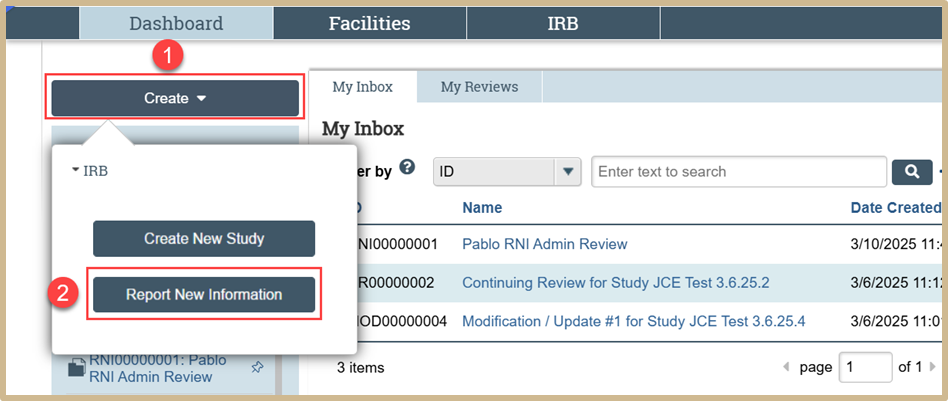
From your Dashboard, click on Create and then select Report New Information from the drop-down menu.
Option #2
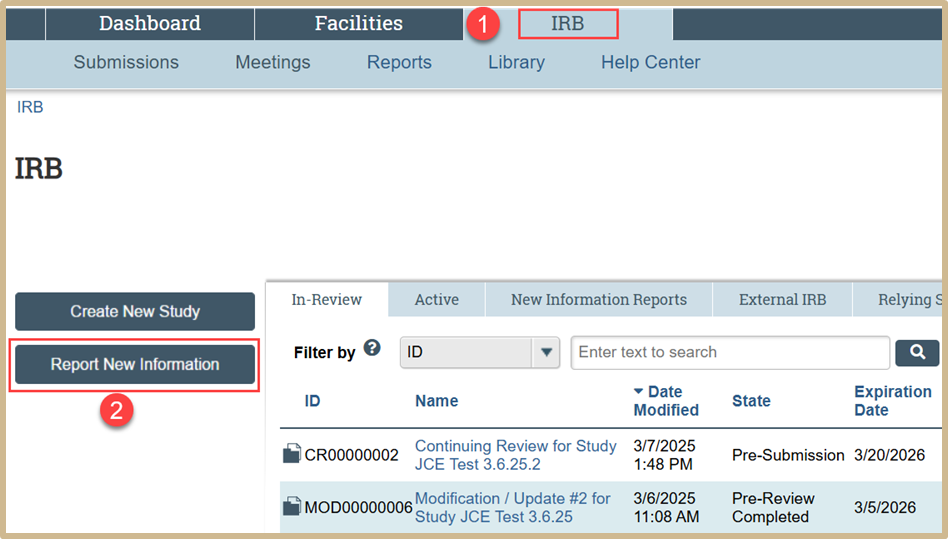
Select the IRB tab from your Dashboard. Then select Report New Information.
Option #3
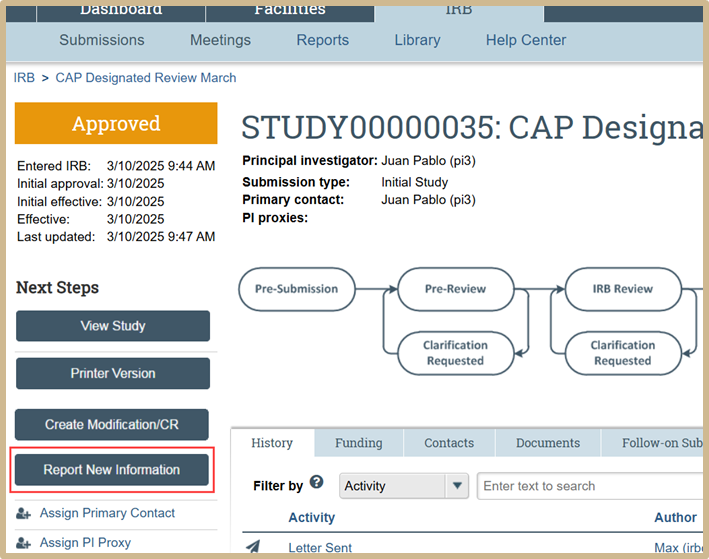
Click on any active study and then select Report New Information.
12.1 Reportable New Information

![]() Select a brief description for this RNI submission that distinguishes it from other submissions. You can use any unique title shorter than 50 characters.
Select a brief description for this RNI submission that distinguishes it from other submissions. You can use any unique title shorter than 50 characters.
The short title identifies the RNI throughout the IRB system, such as in your inbox and in the IRB’s list of submissions to review.
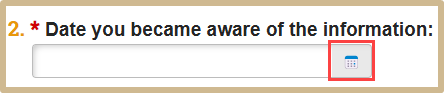
Enter the date you became aware of the information.
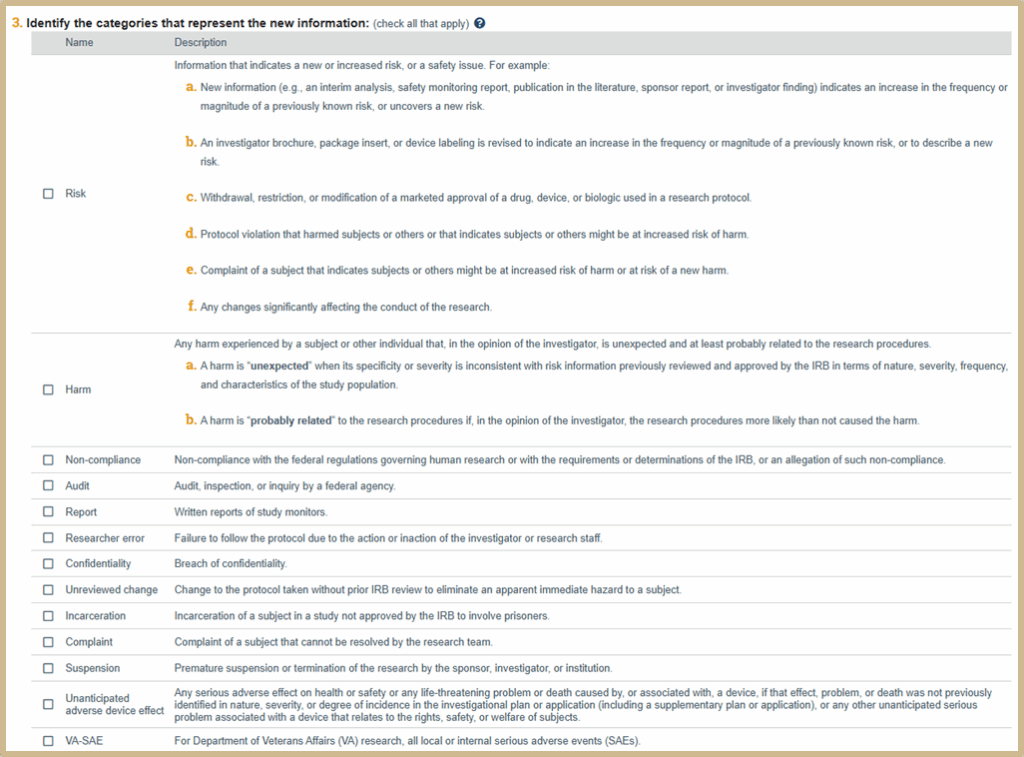
![]() Identify the type of event from the list of categories below. You can select more than one category. Information that does not fit into any of the categories provided does not need to be reported to the IRB as new information.
Identify the type of event from the list of categories below. You can select more than one category. Information that does not fit into any of the categories provided does not need to be reported to the IRB as new information.
![]() Include detailed, relevant information to allow the IRB to make a determination about the reportable information. Specify what occurred, how the event fits into one or more categories above, and any corrective actions the study team has already taken to address the event.
Include detailed, relevant information to allow the IRB to make a determination about the reportable information. Specify what occurred, how the event fits into one or more categories above, and any corrective actions the study team has already taken to address the event.

![]() Select Yes if the information indicates an increase in the frequency or magnitude of a previously known risk or uncovers a new risk or safety issue. For example:
Select Yes if the information indicates an increase in the frequency or magnitude of a previously known risk or uncovers a new risk or safety issue. For example:
- A subject experienced a potential side effect identified in the consent form, but the event is more severe than anticipated.
- A subject experienced an adverse event that is probably related to the research but not previously identified as a risk associated with the study.
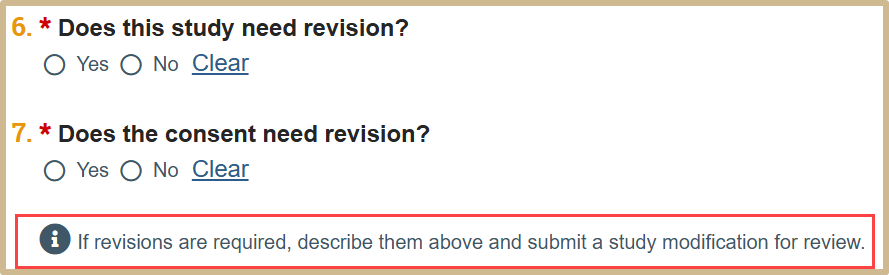
Select if the study or consent needs revision. If any revisions are required, describe them in question #4 and submit a study modification for review.

![]() The PI, PI proxies, and primary contact of each related submission are notified at several points in the new information workflow. In addition, the PI and PI proxies of each related submission can edit the new information and submit it for review, along with submitting a response to a clarification request.
The PI, PI proxies, and primary contact of each related submission are notified at several points in the new information workflow. In addition, the PI and PI proxies of each related submission can edit the new information and submit it for review, along with submitting a response to a clarification request.
The modifications in the list are filtered so only modifications to studies already related to this RNI are shown.
Tip: To add a related modification when it is not available in the selection list:
- Add the modification’s parent study.
- Click the Save link at the bottom of the page.
- Now click Add again under Related studies and modifications.
The modification is available in the selection list.
Attach any files containing supporting information.
When finished, select Continue to move to the Final Page.
12.2 Final Page
Click Finish to exit the form.
12.3 Submit RNI
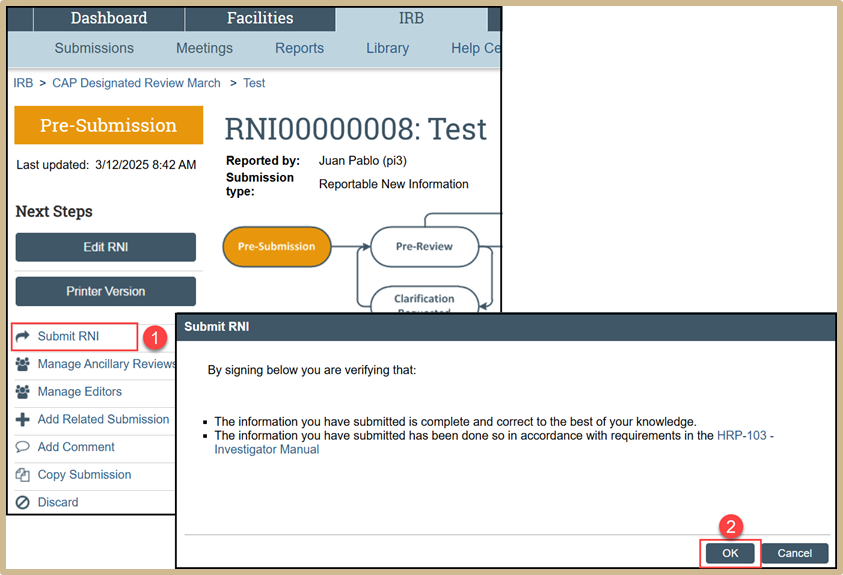
Select Submit RNI. Verify your submission by selecting OK.
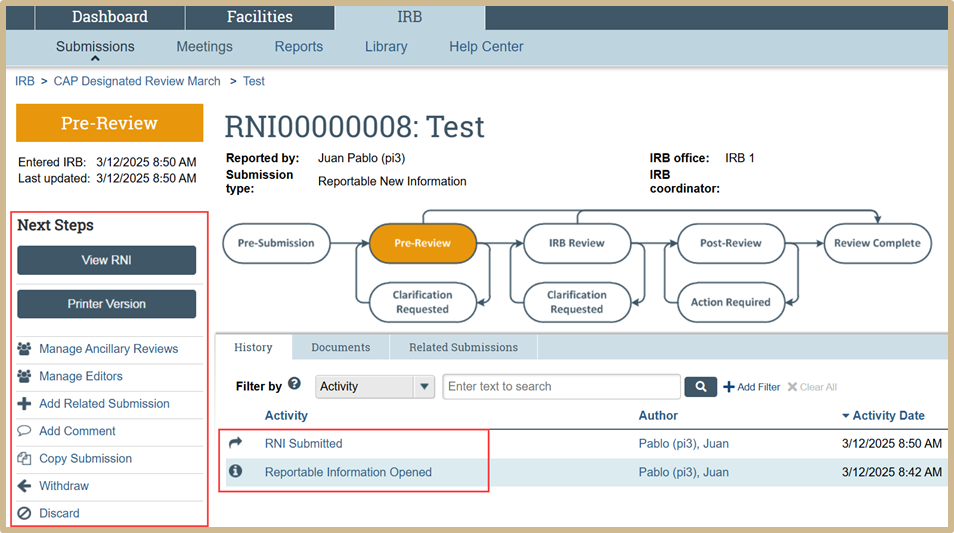
The RNI has now been submitted and is in Pre-Review status. In the Next Steps activities you may view the RNI, add comments, withdraw, etc.
12.4 Assign Coordinator
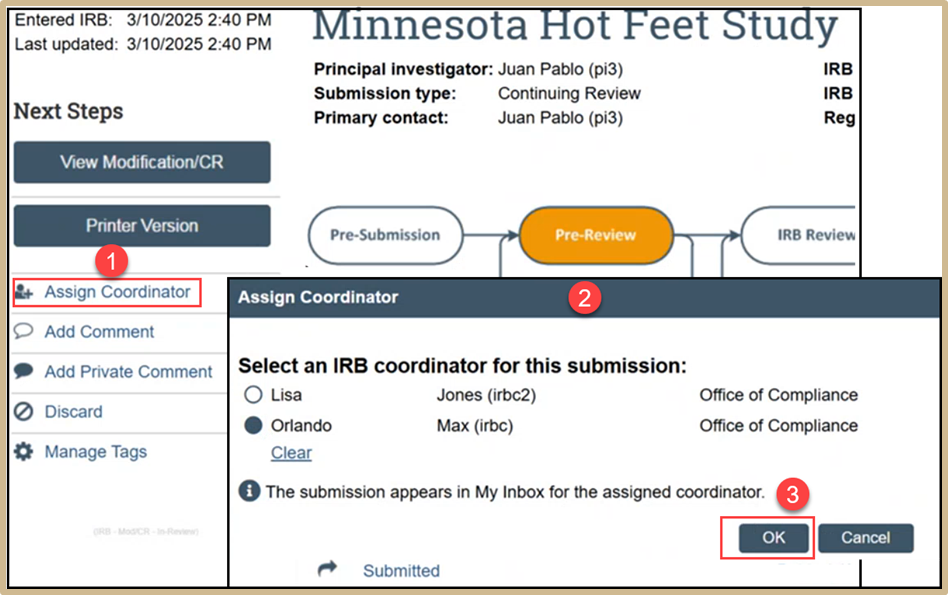
Click on Assign Coordinator and then select an IRB coordinator for the submission. Once finished, select OK.
12.5 View RNIs
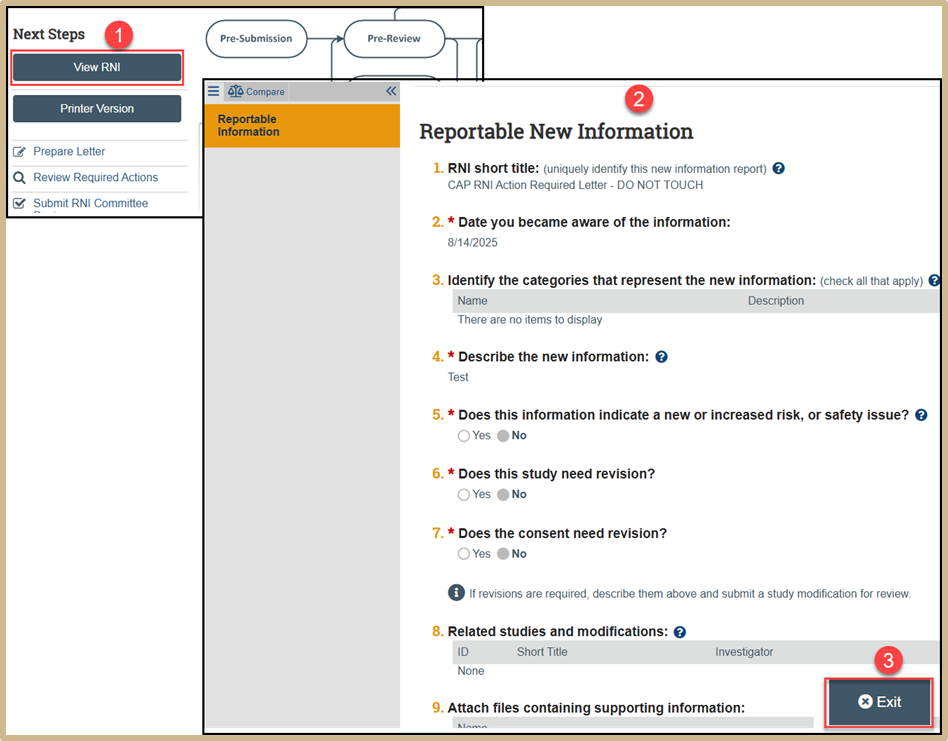
To view the RNI, select View RNI. Review all information. When finished, select Exit.
12.6 Submit RNI Pre-Review
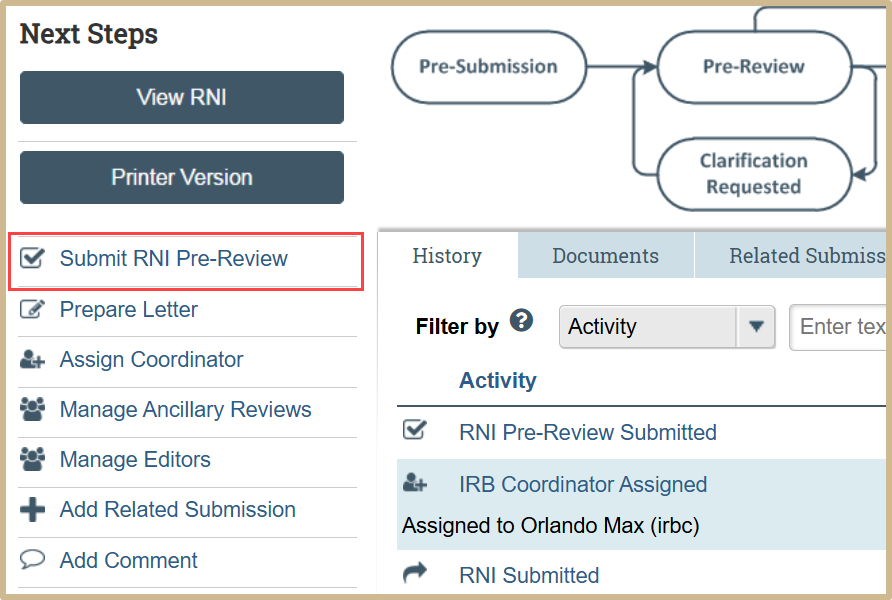
Select Submit RNI Pre-Review from the Dashboard.

![]() Depending on the determinations you select, the new information may move directly to the final state of Acknowledged, or it may require additional review. The rules are as follows:
Depending on the determinations you select, the new information may move directly to the final state of Acknowledged, or it may require additional review. The rules are as follows:
- If you select any of the first four (more significant) determinations from the list or “Additional review required,” the system prepares the new information report to be assigned to a designated reviewer or to committee review.
- Selecting only the less significant determinations and submitting the review causes the new information report to move to either the Post-Review state or directly to the final state of Acknowledged, depending on whether further action is required. If further action is required, the RNI is sent to Post-Review. If further action is not required, the RNI is sent to Acknowledged. If the RNI is sent to Acknowledged, the system sends an automatic e-mail notification to the RNI submitter and the PIs, PI proxies, and primary contacts of all related studies.
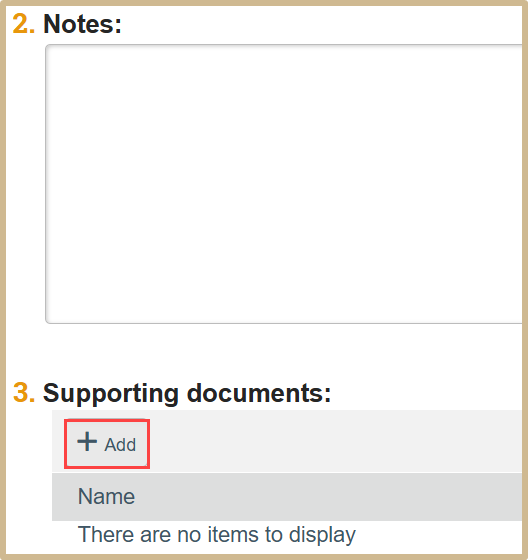
Include any notes or supporting documentation if necessary.
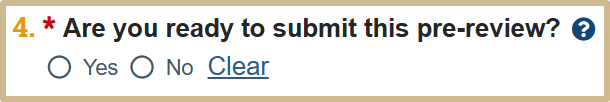
![]() Implications of this choice:
Implications of this choice:
- Yes: Moves the new information report to a final state or the next phase of the workflow, depending on the determinations selected.
- No: Enables you to return and finish the review at another time.
In either case, the information you enter is saved when you click OK.
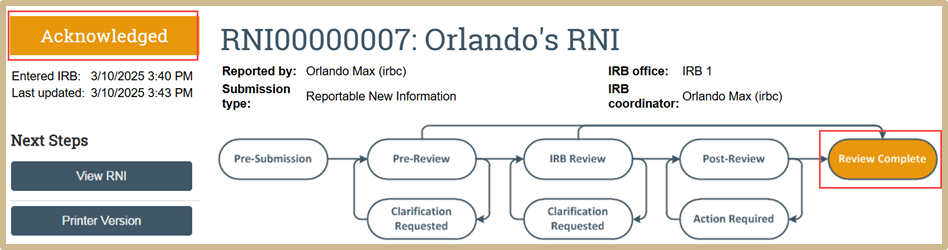
If Yes was selected, the RNI will show that it has been acknowledged and that the review is now complete.
Content Updated: