 PERA IRB Administrative Staff Procedure Guide
PERA IRB Administrative Staff Procedure Guide
3. Study Closure
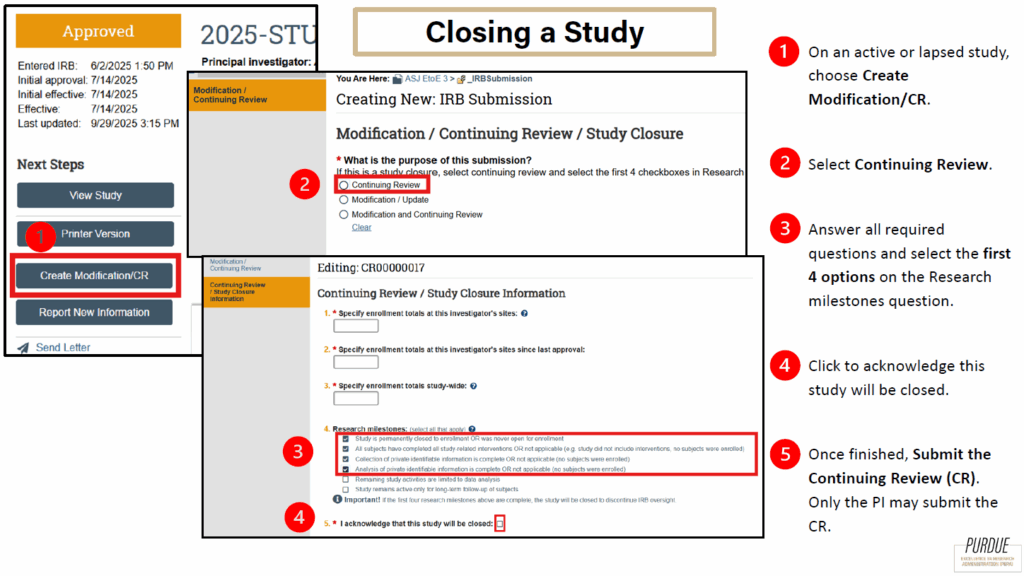

3. Study Closure
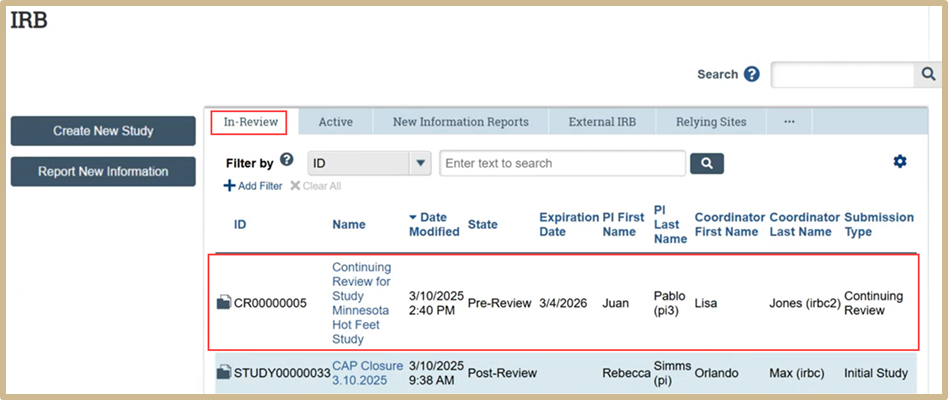
From the IRB tab, select In-Review. Find the study you want to close and select by clicking the study title. Closures will come in as a “CR” number, this number will be different than the Study number.
Just like any other pre-review process, a coordinator needs to be assigned.
3.1 Assign Coordinator (Intake)
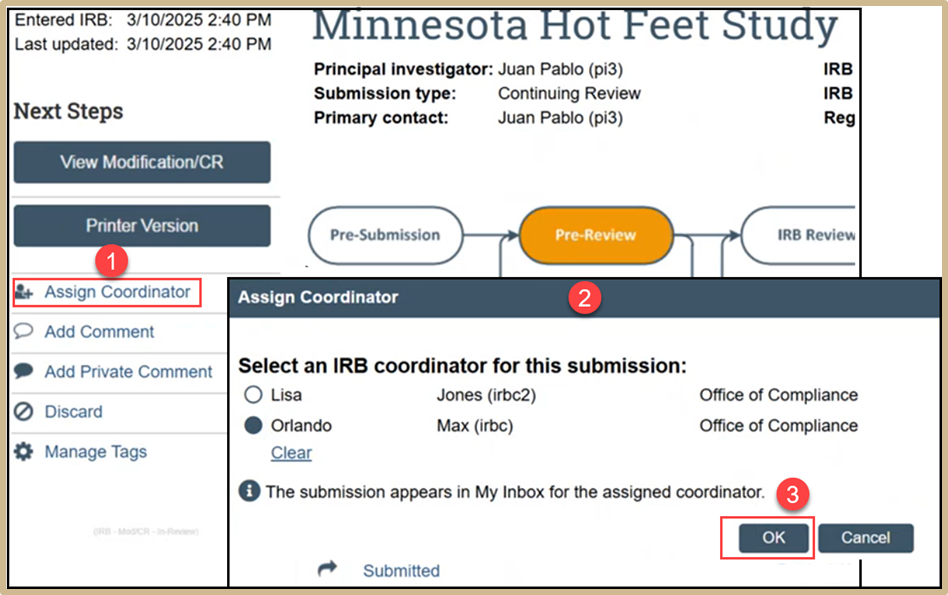
Click on Assign Coordinator if the original coordinator is not already automatically selected. and then select an IRB coordinator for the submission. Once finished, select OK.
3.2 Perform a Pre-Review
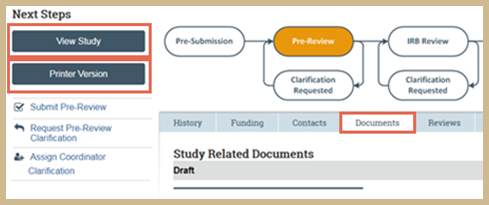
Review the submission and its documents. From the workspace, click the following:
- View Study: Opens the study. Click Continue to move through the pages.
- Printer Version: Shows the study in one scrollable page.
- Documents tab: Shows all attached study documents.
Note: You can also access these documents from the study pages and printer version.
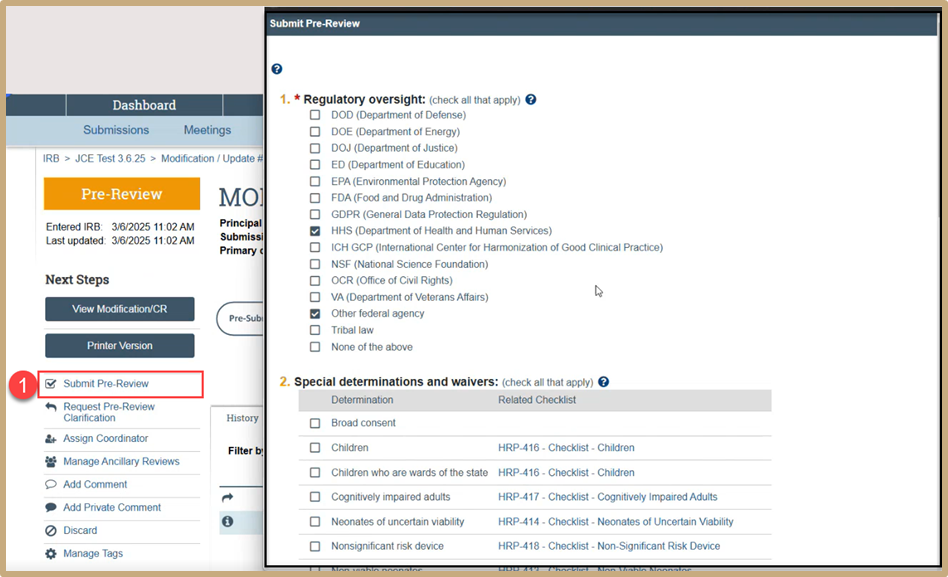
Select Submit Pre-Review. These questions will auto populate based on the original pre-review. You can go through and make any updates as needed, or you can just submit the pre-review if there are no changes.
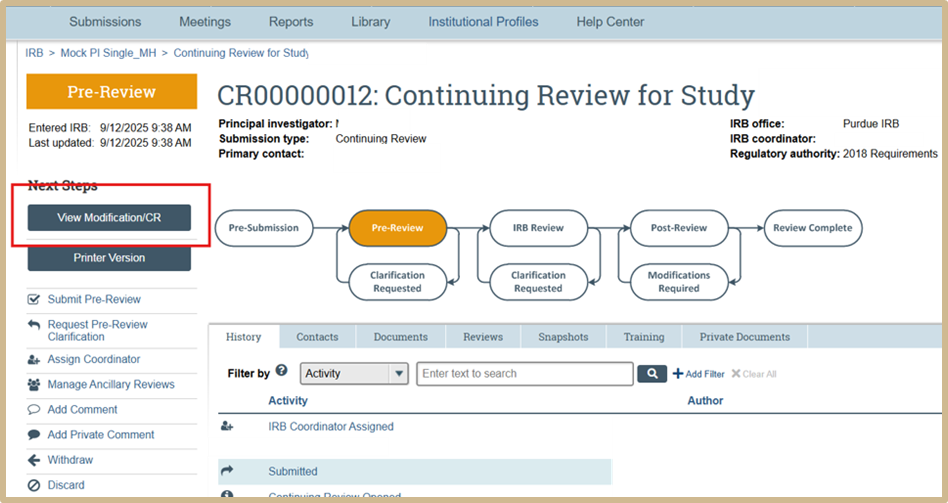
To review the original pre-review CR select View Modification/CR.

Select Yes once you are ready to submit the pre-review and then click OK to finish.
3.3 Assign Designated Reviewer
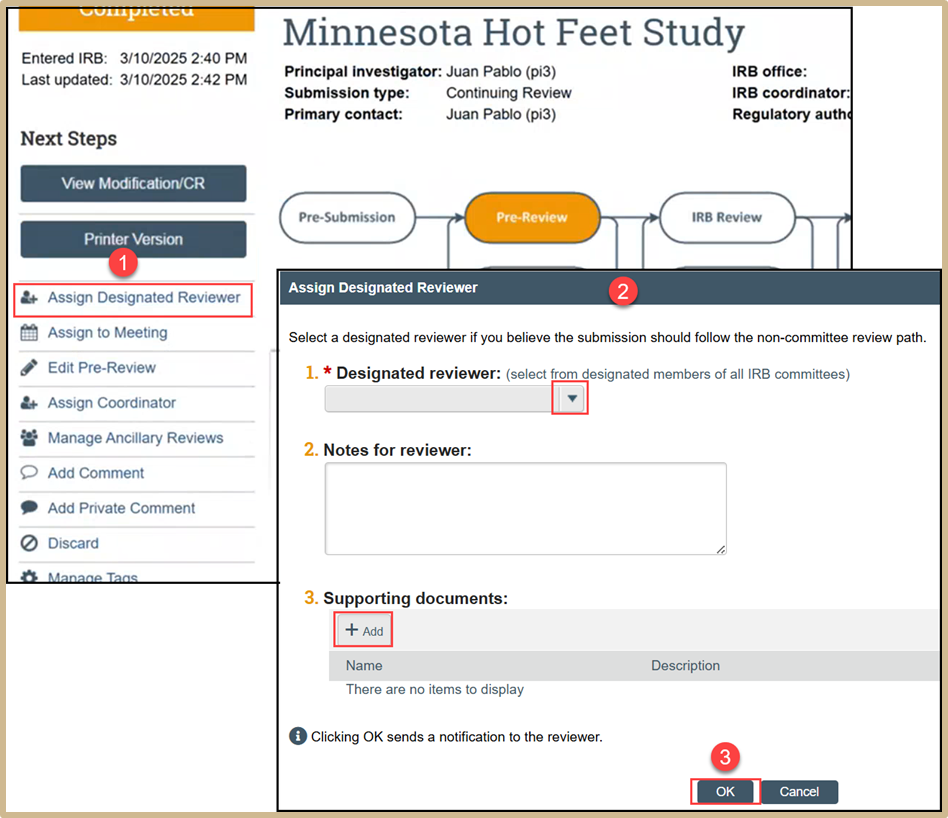
Select Assign Designated Reviewer. Select a designated reviewer if you believe the submission should follow the non-committee review path. Add any supporting documentation as needed. Click OK to send a notification to the reviewer.
3.4 Submit Designated Review
A coordinator can be assigned as the designated reviewer.
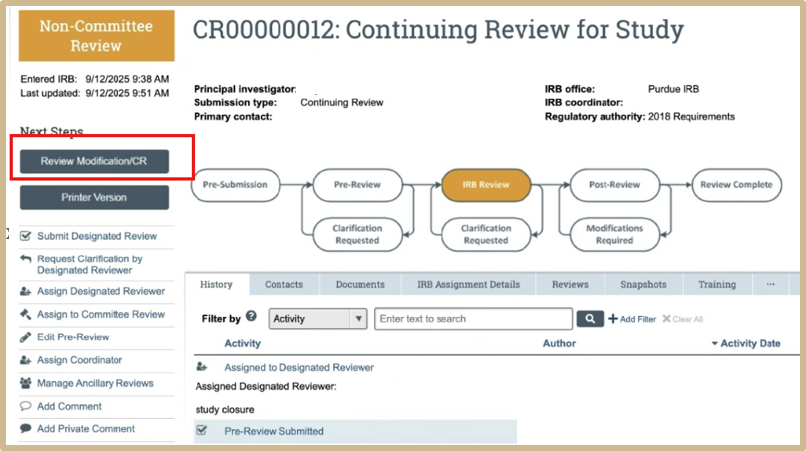
The designated reviewer needs to review first. Select Review Modification/CR. Review all information before submission.
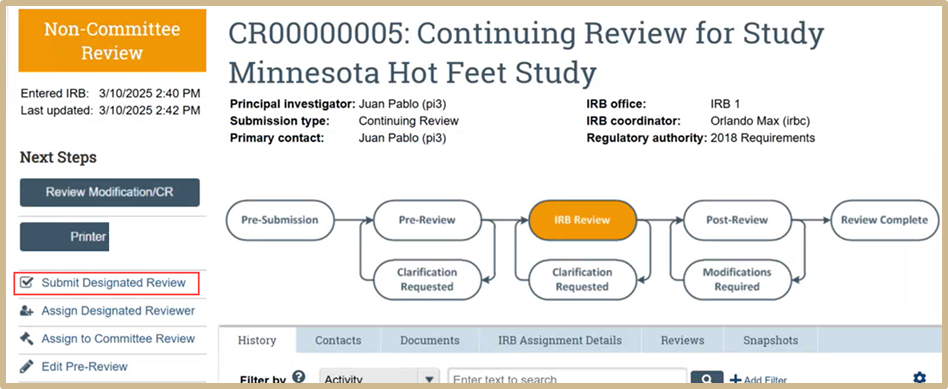
Select Submit Designated Review.
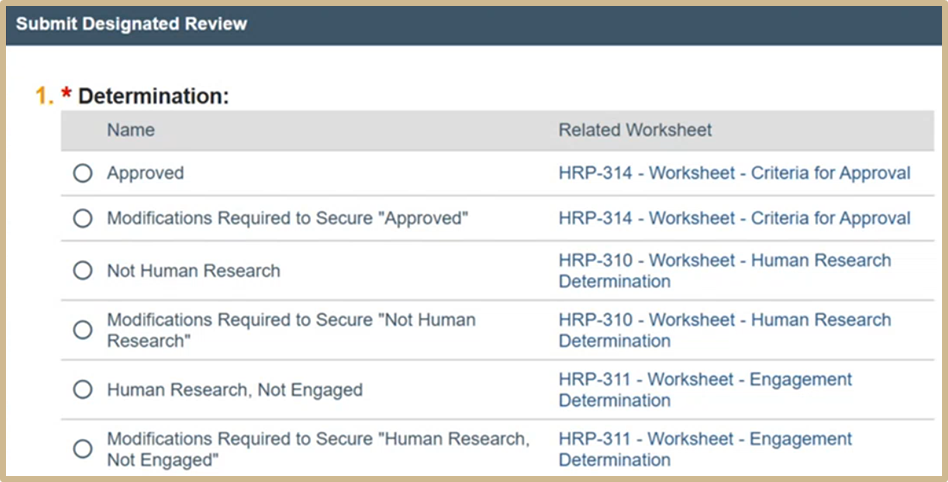
Select Approved to move study to closure.

![]() Minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests (from subpart A).
Minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests (from subpart A).
Note: For research involving prisoners, minimal risk is the probability and magnitude of physical or psychological harm that is normally encountered in the daily lives, or in the routine medical, dental, or psychological examination of healthy persons (from subpart C).
Select Risk level.
![]() Only if Approved is chosen in question #1
Only if Approved is chosen in question #1
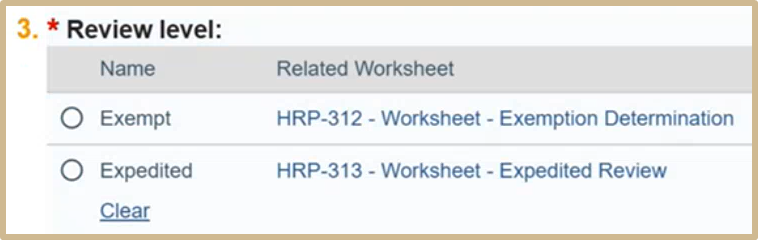
Choose the Review level.

Check all categories that apply. If the category is not listed, please select Other.
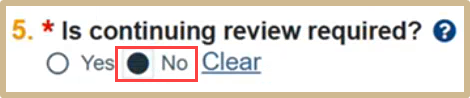
![]() Continuing Review is not required for minimal risk research that falls under the 2018 Common Rule requirement.
Continuing Review is not required for minimal risk research that falls under the 2018 Common Rule requirement.
Select No since you are closing out the review.
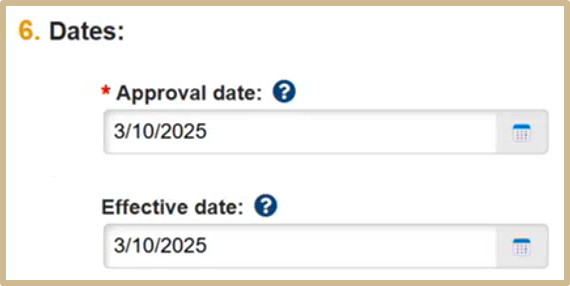
![]() Approval Date: The date the IRB decision was made. If you are submitting a committee review, the date defaults to the day of the meeting. It must be set to the day the committee made the decision, not the day the decision is recorded.
Approval Date: The date the IRB decision was made. If you are submitting a committee review, the date defaults to the day of the meeting. It must be set to the day the committee made the decision, not the day the decision is recorded.
Effective Date: The date the IRB decision takes effect.
For initial submissions, this is typically the start day of the approval period.
The effective date is most significant when modifications are required before approval, because the research cannot start immediately when the IRB decision is made (the approval date). The effective date is set to the day the modifications are reviewed and accepted. The last day of approval can be set based on the effective date instead of the approval date.
Enter study closure into the textbox in question #7.

Checkmark the box stating that you do NOT have a conflicting interest.
![]() Check the box if you have no conflicting interest with reviewing this research.
Check the box if you have no conflicting interest with reviewing this research.
If you have a conflicting interest, you cannot perform the review. In that case, click the Cancel button at the bottom of the form. Then contact the IRB coordinator listed near the top of the screen to request reassignment of the review.
Definition of conflicting interest:
Who holds the interest? The individual involved in the review, or the immediate family of the reviewer. Immediate family means the spouse, domestic partner, children, or dependents.
Interest in what? The study sponsor, a competitor of the sponsor, a product or service being tested, or a competitor of the product or service being tested.
What is an interest? Any of the following:
- Involvement in the design, conduct, or reporting of the research.
- Ownership interest of any value including (but not limited to) stocks and options, exclusive of interests in publicly-traded, diversified mutual funds.
- Compensation of any amount in the past year or expected in the next year, including costs directly related to conducting research.
- Proprietary interest including (but not limited to) patents, trademarks, copyrights, or licensing agreements.
- Any other reason for which you believe you cannot be independent.

![]() Implications of this choice:
Implications of this choice:
- Yes: Moves the submission to the Post-Review state so the investigator can be informed of the determination.
- No: Enables you to return and finish the review at another time (by clicking the activity again).
In either case, the information you enter is saved when you click OK.
Select Yes when you are ready to submit and close out the review.
When finished select OK.
3.5 Post-Review Activities
After the designated member or committee review decision has been submitted for a submission, the next steps are to finalize the documents and prepare and send the determination letter to PI.
To finalize documents
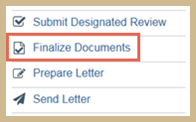
- From the study workspace, click Finalize Documents.
- Select the documents to change to PDF and watermark.
- Click OK.
The Documents tab on the study workspace will include links to the final versions of the documents.
To prepare determination letter
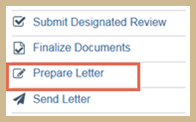
- From the study workspace, click Prepare Letter.
- You have two options:
- To create a letter from a template, select the template from the list and click Generate.
Note: You can open the draft letter, change it as needed, and then add the revised document.
- To add a letter, click Upload and then browse for the letter.
- Click OK when done.
Until you send the letter, you can use the Prepare Letter activity to regenerate the letter again or upload revisions.
To send determination letter
- From the study workspace, click Send Letter.
- Review the determination and letter and then click OK.
Note: You may want to verify the accuracy of the dates listed on the Send Letter page before clicking OK.
The letter is sent to the PI, PI proxy, and primary contact.
3.6 Prepare Study Closure Letter
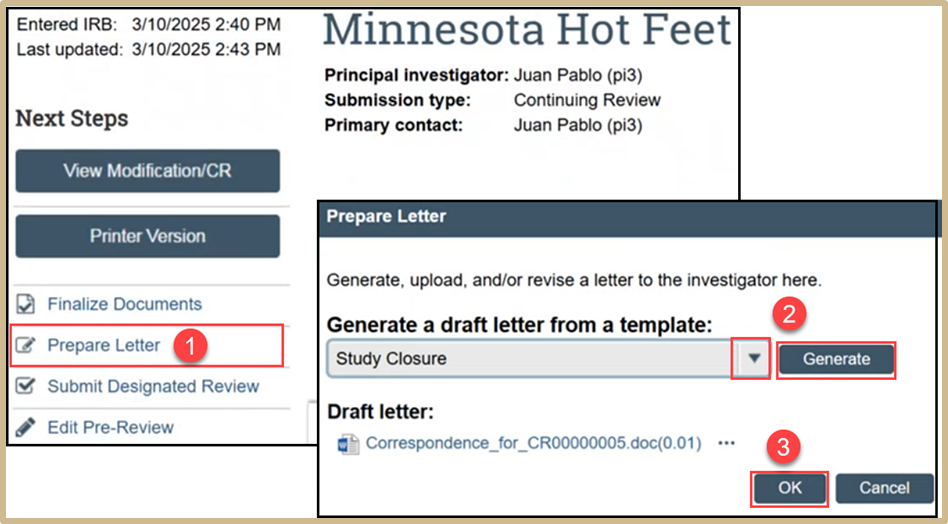
Select Prepare Letter to initiate a study closure letter. Click the drop-down arrow and select Study Closure. Select Generate and a word document will appear under Draft letter. When finished, click OK.
3.7 Send Study Closure Letter
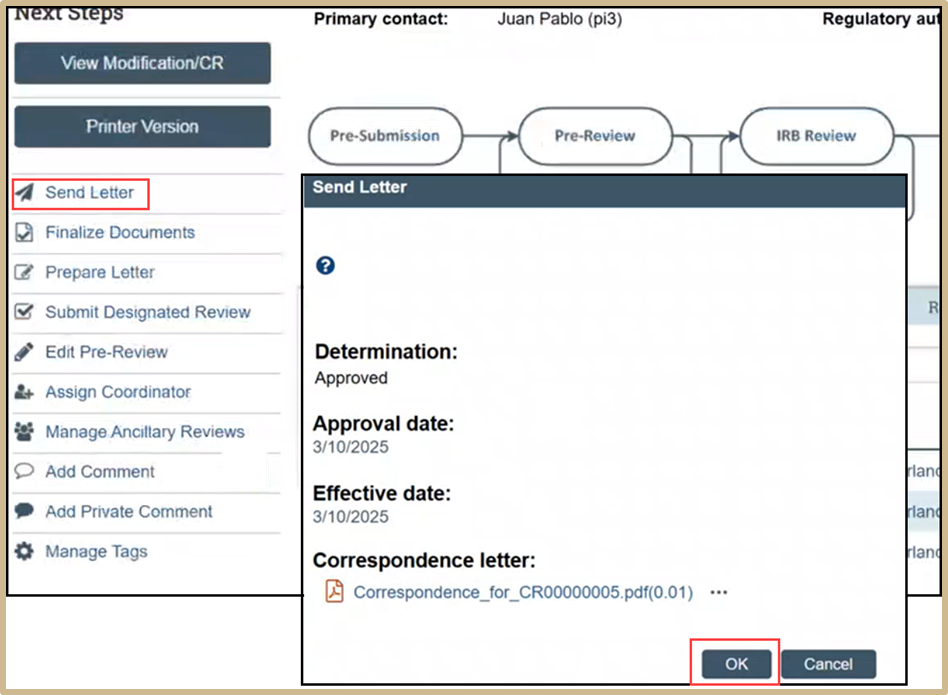
Select Send Letter. Review the information and then press OK.
NOTE: If there is Psite, the Psite will need to be closed first before you can send the study closure letter.
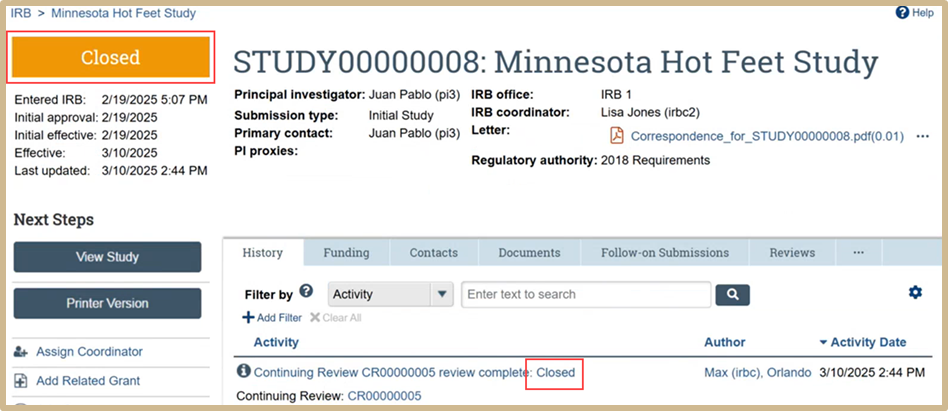
The study is now Closed.
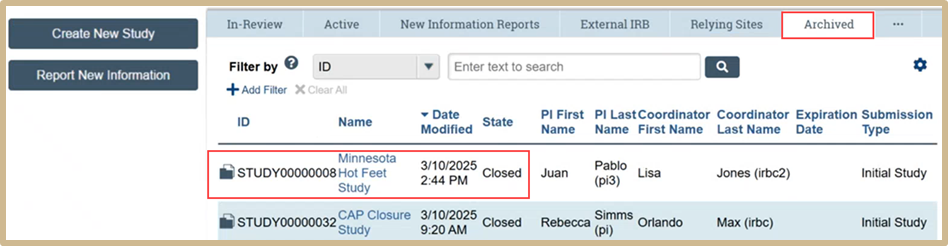
Once the study is closed it can be viewed in the Archived folder.
Content Updated: