Create a Protocol


Create a Protocol
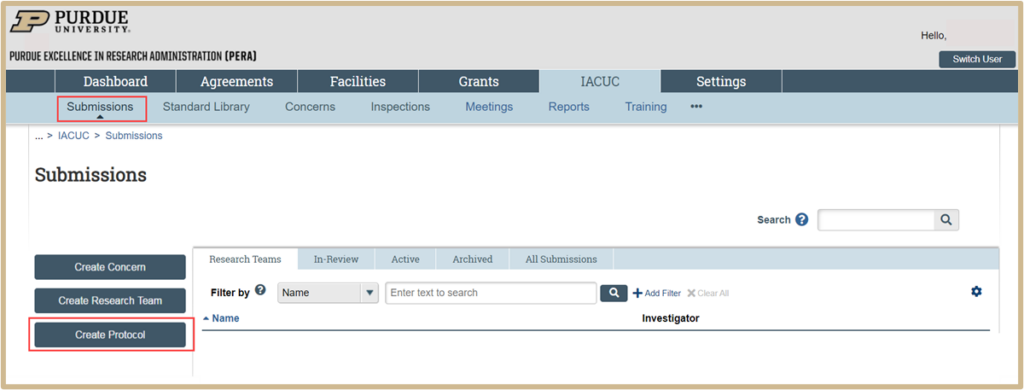
Click Create Protocol under the Submissions tab then proceed through the steps below.
Basic Information and Funding

This section will review the forms for Basic Information & Funding, which includes:
Basic Information
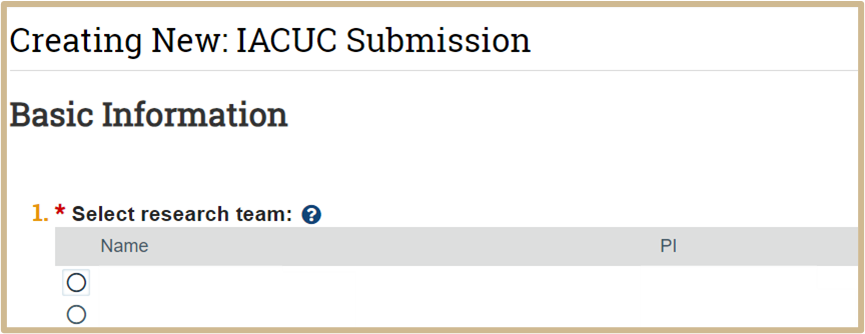
![]() If you are on more than one animal use team, select it in the list. If the research/animal use team does not appear in the list, you will need to create it first before creating the protocol. Return to the Dashboard and use the Create Research Team activity on the left.
If you are on more than one animal use team, select it in the list. If the research/animal use team does not appear in the list, you will need to create it first before creating the protocol. Return to the Dashboard and use the Create Research Team activity on the left.
![]() NOTE
NOTE
Research/animal use team refers to all Animal Use Teams, not just Research.

Provide a title of your protocol.

![]() Providing a Short Title is a Huron Software Solutions requirement. Type a short title for your protocol. You can use a sponsor’s short title, any other unique name, or the same title that you used in #2 above, as long as it is no more than 50 characters. The maximum for the Short Title is 50 Characters.
Providing a Short Title is a Huron Software Solutions requirement. Type a short title for your protocol. You can use a sponsor’s short title, any other unique name, or the same title that you used in #2 above, as long as it is no more than 50 characters. The maximum for the Short Title is 50 Characters.
The short title identifies the protocol throughout the IACUC system, for example, in My Inbox.

![]() Briefly state the objective(s) for this use of animals. Use terminology that can be understood by someone with minimal knowledge of the specific use of animals.
Briefly state the objective(s) for this use of animals. Use terminology that can be understood by someone with minimal knowledge of the specific use of animals.
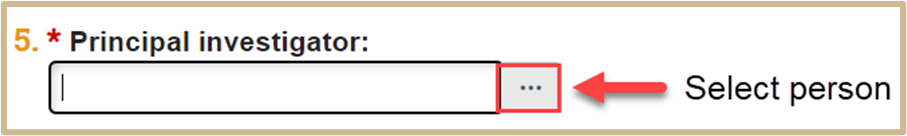
Identify the Principal Investigator.
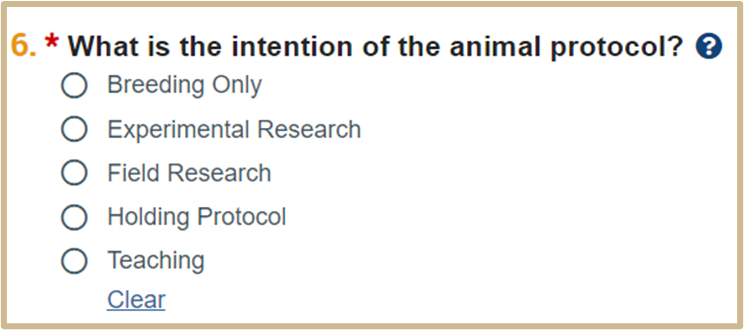
![]() Select the option that BEST reflects the nature of the protocol. What you select here will affect the remaining pages that appear for this protocol.
Select the option that BEST reflects the nature of the protocol. What you select here will affect the remaining pages that appear for this protocol.
IMPORTANT
If you are doing experimental research with breeding, select Experimental Research. On the next page, you can indicate that the research includes breeding.
If this is a teaching protocol, you must attach written documentation as to what is provided to students enrolled in the course informing them of potential personal risk when working with animals (e.g., zoonotic disease, allergens, biohazard exposure, kicks, bites, etc.) and how students are informed of IACUC’s policy on How to Report Concerns. Students should be told the following:
Any concerns about animal use or health can be reported anonymously to the IACUC office by calling the Purdue Hotline, 1-866-818-2620. They may also email the IACUC office to report concerns at iacuc@purdue.edu. The animal Safety Verification Form provided to you for this protocol must also be shared with all students enrolled in your course.
![]() This is only applicable if Experimental Research was chosen as the intention of the animal protocol in Step 6 above.
This is only applicable if Experimental Research was chosen as the intention of the animal protocol in Step 6 above.

![]() Indicate if the protocol will include breeding. The answer you specify here will affect the remaining pages that appear for this protocol.
Indicate if the protocol will include breeding. The answer you specify here will affect the remaining pages that appear for this protocol.
Protocol Team Members

![]() When creating the protocol, the PI’s team will list by default when the team is chosen in #1 above, but you can add and delete protocol team members if necessary. If your research team was created during migration from Coeus to PERA, all team members will have Involved in Animal Handling and Authorized to Order Animals marked “YES”. If you would like to change either of these to “NO” for a team member, you will need to click on their name and change the answer.
When creating the protocol, the PI’s team will list by default when the team is chosen in #1 above, but you can add and delete protocol team members if necessary. If your research team was created during migration from Coeus to PERA, all team members will have Involved in Animal Handling and Authorized to Order Animals marked “YES”. If you would like to change either of these to “NO” for a team member, you will need to click on their name and change the answer.
![]() NOTE
NOTE
-The PI is specified on the Basic Information page. Do not add that person again here.
-Only the PI, PI proxy, and people on the protocol team can view and edit the protocol. If an animal use team member is not a protocol team member, that person will not be able to access the protocol.
-If the primary contact is engaged in the animal use activity, be sure to add that person to the protocol team.
Once finished, click OK or OK and Add Another.
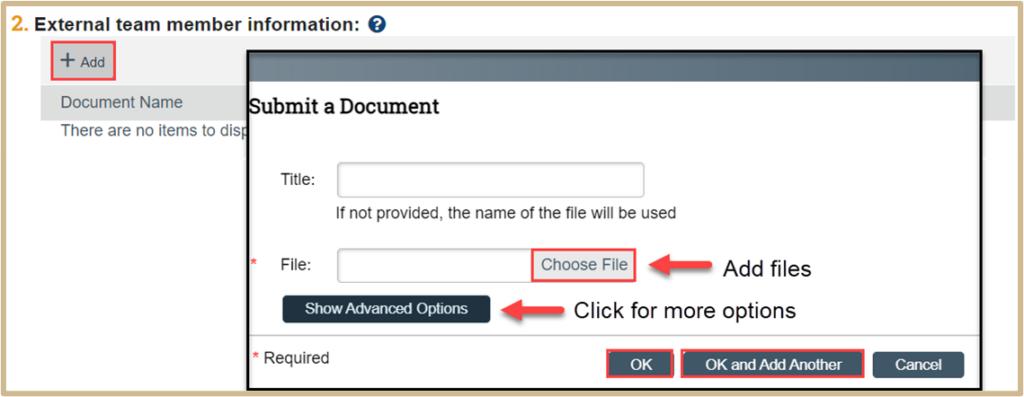
![]() Attach information about protocol team members that are not in the IACUC system (the person is not available for selection in the previous question). External members could be people outside your institution, such as consultants or collaborators who may be working on your animal use protocol. These would be people not in Purdue’s HR system. This is where Training Exemption Forms should be uploaded for those working on your animal use protocol, but are not in the Purdue HR system.
Attach information about protocol team members that are not in the IACUC system (the person is not available for selection in the previous question). External members could be people outside your institution, such as consultants or collaborators who may be working on your animal use protocol. These would be people not in Purdue’s HR system. This is where Training Exemption Forms should be uploaded for those working on your animal use protocol, but are not in the Purdue HR system.
IMPORTANT
Do not attach information about team members you were able to select in the previous field. For people listed in the system, member information should be added to their profiles instead. If you are unsure how to proceed, contact your IACUC staff for assistance.
Select Continue to proceed to Funding Sources.
Funding Sources
 Only complete this section if the source is funded, otherwise, leave it blank and move on to the next step, Experimental Design.
Only complete this section if the source is funded, otherwise, leave it blank and move on to the next step, Experimental Design.

![]() Identify all funding sources, such as industry sponsors and government agencies. The main purpose is to help the IACUC identify all protocols associated with particular grants. If funding comes from a specific internal funding program, also identify that funding source.
Identify all funding sources, such as industry sponsors and government agencies. The main purpose is to help the IACUC identify all protocols associated with particular grants. If funding comes from a specific internal funding program, also identify that funding source.
Click +Add to identify each organization that is supplying funding for the protocol.
In the new pop up window, select the funding organization. You can also include the Sponsor’s funding ID which is assigned by the external sponsor, Grants office ID that is assigned internally, and attach any files including any grant applications as needed.
Once finished, click OK or Ok and Add Another.

Select Continue to proceed to Experimental Design.
Experimental Design

The following items need to be completed in the Experimental Design section:
Scientific Aims
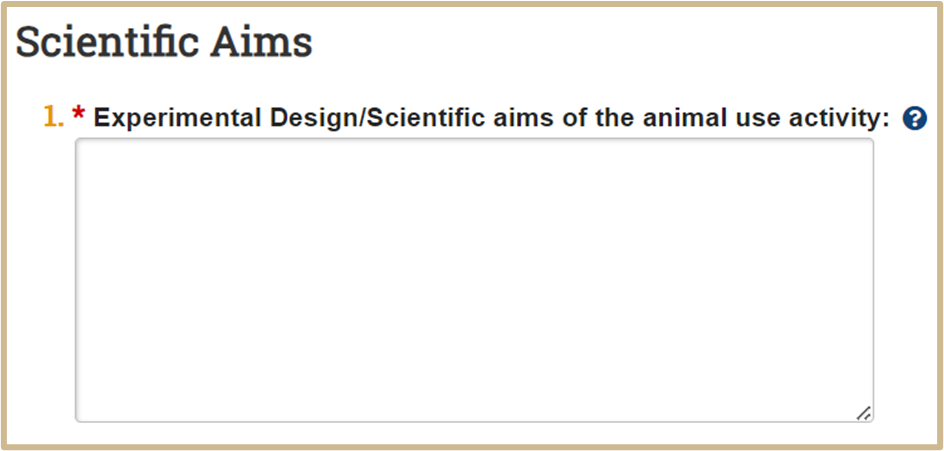
![]() Type the goals or objectives of the animal use activity.
Type the goals or objectives of the animal use activity.
Briefly explain the experimental design. Please list the name of all animal procedures you will perform. All procedures, non-surgical and surgical, to be performed in the study/activity must be listed.
For Example:
Procedures to be performed:
Food/Fluid Restriction
Imaging
Anesthesia
Hind-limb Surgery
Euthanasia
![]() NOTE
NOTE
Surgical details MUST be specified in a non-survival surgery or survival surgery Procedure form, so there is no need to provide detail in this section, just the name of the surgery. This description should allow the IACUC to understand the experimental course of an animal from its entry into the study/activity to the endpoint of the study/activity. A flowchart may be an effective presentation of your experimental design. Please use terms that can be understood by those not familiar with your area of expertise.
You must include a narrative of how the procedures performed could have an impact on the animals’ health and well-being.
Tables, flow charts, graphs, etc., MUST be added to the Supporting Documents tab. They cannot be embedded in the SmartForm.
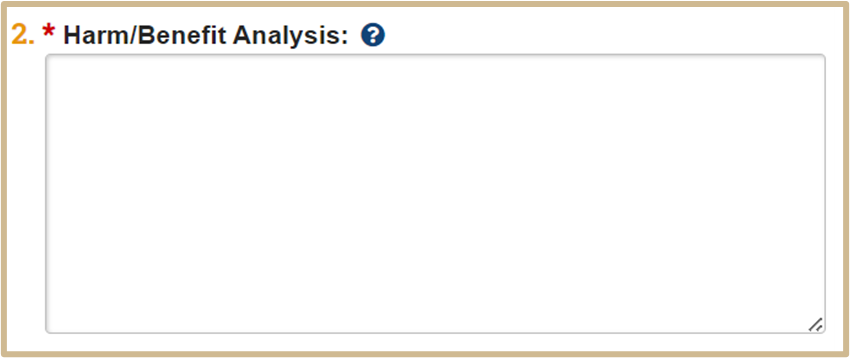
![]() Explain why the animal use activity is important and how society could benefit from the activity (e.g., improvement to human or animal health, advancement of knowledge, etc.), and how the benefits of the activity described in this protocol outweigh any pain and/or distress that may be caused to the animals.
Explain why the animal use activity is important and how society could benefit from the activity (e.g., improvement to human or animal health, advancement of knowledge, etc.), and how the benefits of the activity described in this protocol outweigh any pain and/or distress that may be caused to the animals.
Helpful Websites:
https://www.na3rsc.org/best-practices/
https://felasa.eu/working-groups/reports/id/40
Select Continue to proceed to Experiments.
Experiments
![]() Use this section to add all your experiments to the protocol. When you add an experiment, you will specify the number of animals used in the experiment. You will also select the procedures that apply and identify the variations to the selected procedures. A team procedure used in at least one approved protocol is prefixed with a check mark.
Use this section to add all your experiments to the protocol. When you add an experiment, you will specify the number of animals used in the experiment. You will also select the procedures that apply and identify the variations to the selected procedures. A team procedure used in at least one approved protocol is prefixed with a check mark.
TIP
If your experiments are similar, speed up the process by adding the first one, then copying and making changes to the copy:
1. Click Copy to the right of the experiment you want to copy.
2. Click the experiment name ending in ” – Copy” and make the appropriate changes
3. Click OK
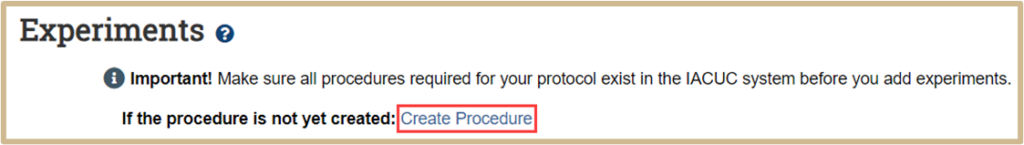
Click on Create Procedure if you have not created one yet for the Experiment.
![]() NOTE
NOTE
When you create a procedure from the experiments screen, it will be added to your team’s library of procedures for later use.
Once you have added experiments and related procedures, the experiments table includes links to edit the experiment and view procedure details.

Select +Add to define in detail the experiments to be used in the protocol.
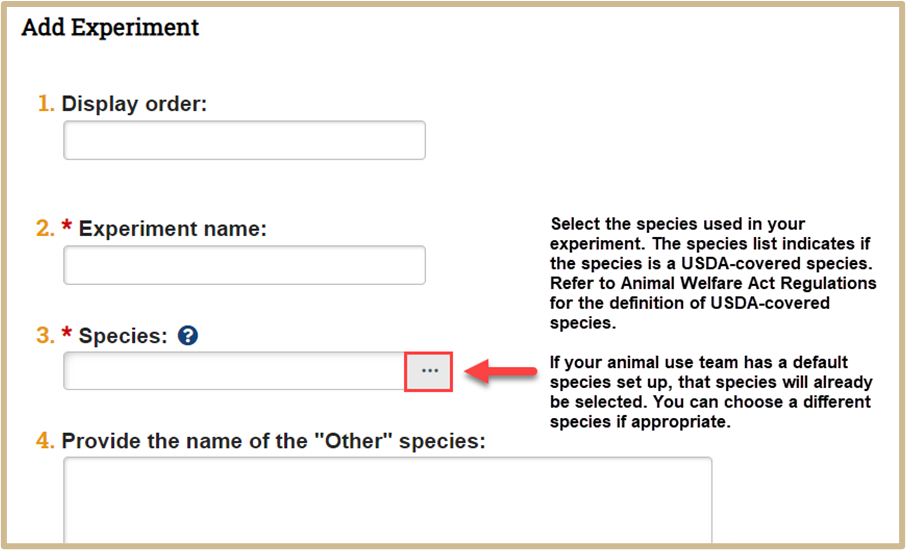
In the new popup window, complete the following questions. Display order can be used to prioritize the listing order of protocol experiments.
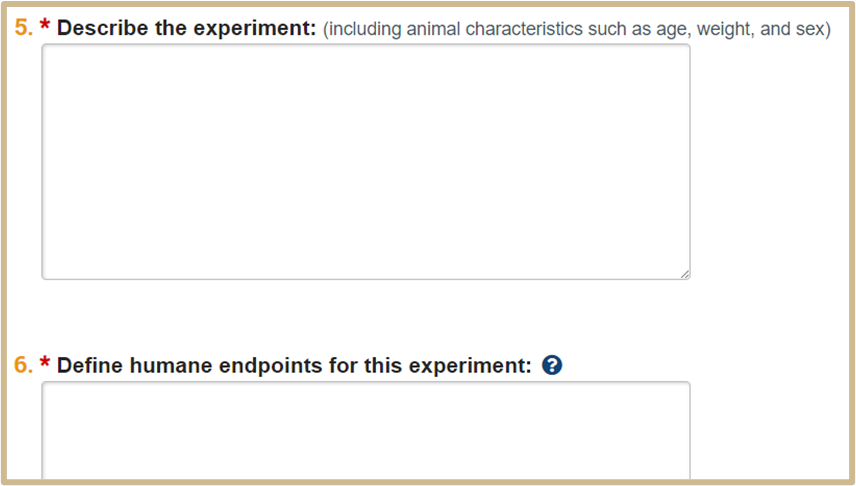
![]() For Question 6
For Question 6
Always add the following statement: See the Humane Endpoint Template uploaded in #15 Supporting Documents.
Then, remember to upload the appropriate Humane Endpoint Template from the IACUC website in Question 15.
Visit Animal Research Forms, SOPs and Guidelines to learn more.

![]() Add the procedures that will be conducted on the animals in the experiment. The list of procedures that appears contains only those procedures for the species in the experiment.
Add the procedures that will be conducted on the animals in the experiment. The list of procedures that appears contains only those procedures for the species in the experiment.
Tip
You can start typing text, and the system will list all procedures that contain the typed text. If a procedure does not appear in the list, go back to the Experiments page and use the Create Procedure link at the top to add it.
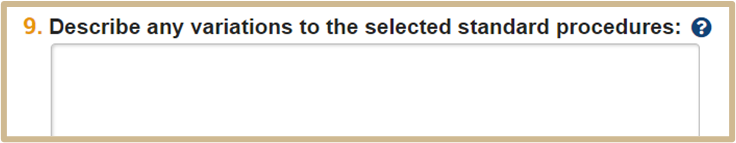
![]() List and explain the variations applied to the procedures. For example, in a substance administration procedure where the dose (variable) will differ across animals, list the dose along with the number of animals that will receive each dose.
List and explain the variations applied to the procedures. For example, in a substance administration procedure where the dose (variable) will differ across animals, list the dose along with the number of animals that will receive each dose.
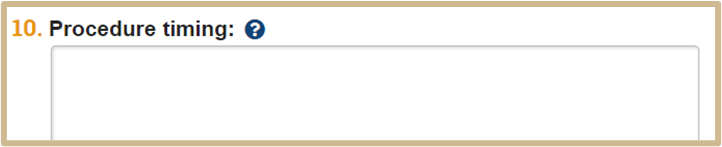
![]() Specify the order procedures will occur and the interval between performing them.
Specify the order procedures will occur and the interval between performing them.
For example:
Perform the following procedures in the order listed. Each procedure is performed immediately after the previous one is completed. Repeat for each animal in the experiment.
Procedure 1. Aerosol administration of agents
Procedure 2. Non-invasive Plethysmography (Pulmonary Function Testing)
Procedure 3. Invasive pulmonary function testing

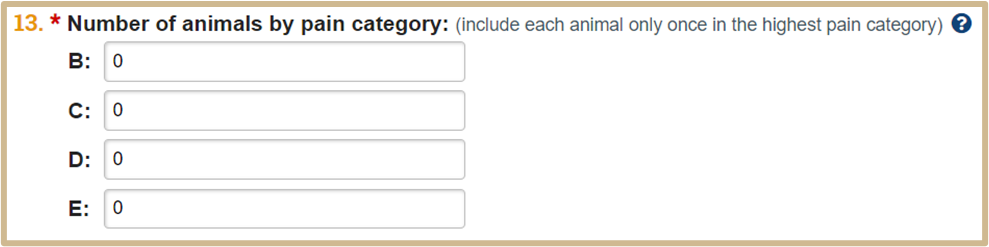
![]() Type the number of animals in each USDA pain category. Include each animal in only one pain category, the highest pain category applicable. The numbers must add up to the total number of animals in the experiment (Question 11). View the USDA Pain Categories for more information.
Type the number of animals in each USDA pain category. Include each animal in only one pain category, the highest pain category applicable. The numbers must add up to the total number of animals in the experiment (Question 11). View the USDA Pain Categories for more information.
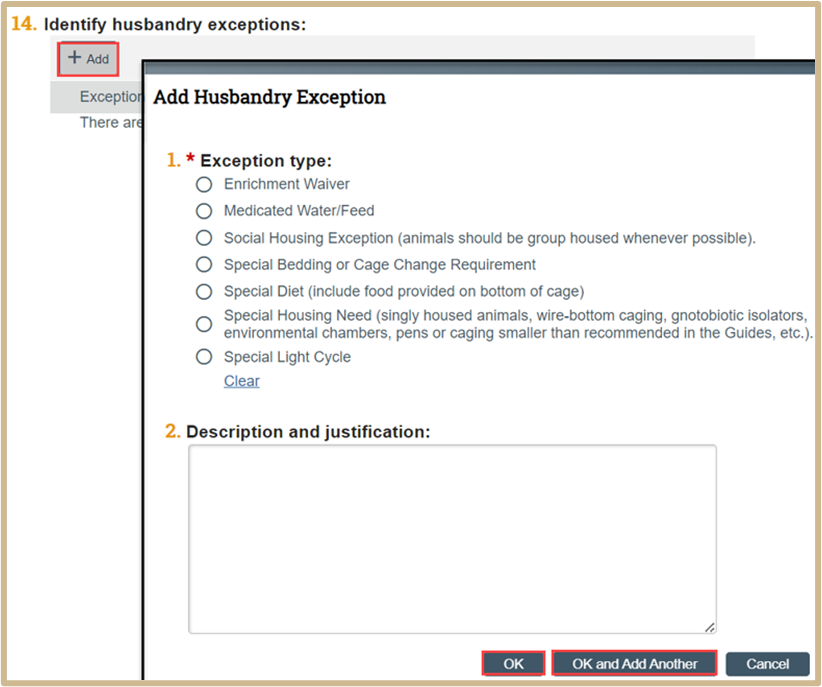
Select +Add to identify husbandry exceptions.
Fill in the questions in the new popup window.
Once finished, click OK or OK and Add Another.

![]() Add any information related to the experiments, for example:
Add any information related to the experiments, for example:
-Methods of alleviating pain and distress, monitoring criteria, and schedules
-Details about husbandry exceptions, exception schedules, monitoring criteria, or weight loss justification
These documents will be listed on the Documents tab of the protocol workspace.
Click +Add to attach any supporting documents.
Fill in the questions in the new popup window.
Once finished, click OK or OK and Add Another.

For Question 2
Answer Yes if any animals undergoing survival surgery fall into one or more of the following categories:
- An animal that underwent surgery performed by a vendor prior to use on this protocol and will undergo survival surgery on this protocol.
- An animal that underwent surgery on another previously approved protocol prior to use on this protocol and will undergo survival surgery on this protocol.
- An animal that will undergo more than one survival surgery procedure at different times during this protocol.
Select Continue to proceed to Procedure Personnel Assignment.
Procedure Personnel Assignment
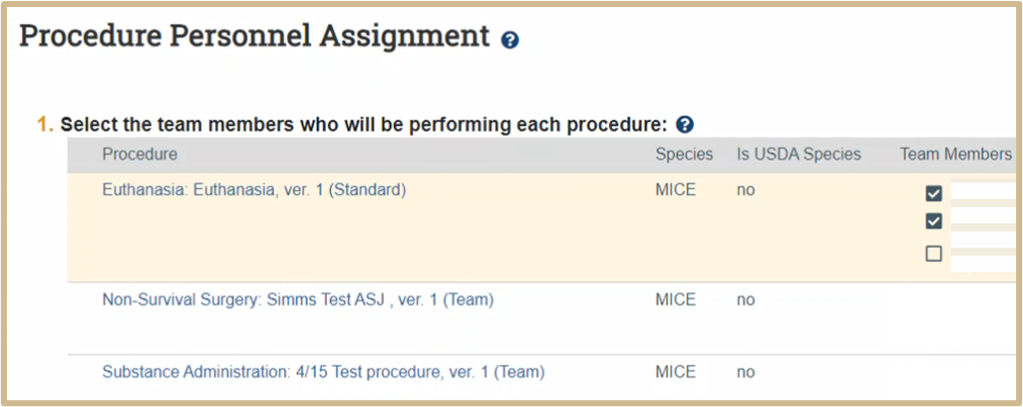
![]() Use this page to assign protocol team members to the procedures used in the experiments. You must assign at least one member to each procedure.
Use this page to assign protocol team members to the procedures used in the experiments. You must assign at least one member to each procedure.
![]() NOTE: Review the training information below to ensure protocol team members have the necessary training before assigning them to a procedure.
NOTE: Review the training information below to ensure protocol team members have the necessary training before assigning them to a procedure.

Select Continue to proceed to Strains.
Strains
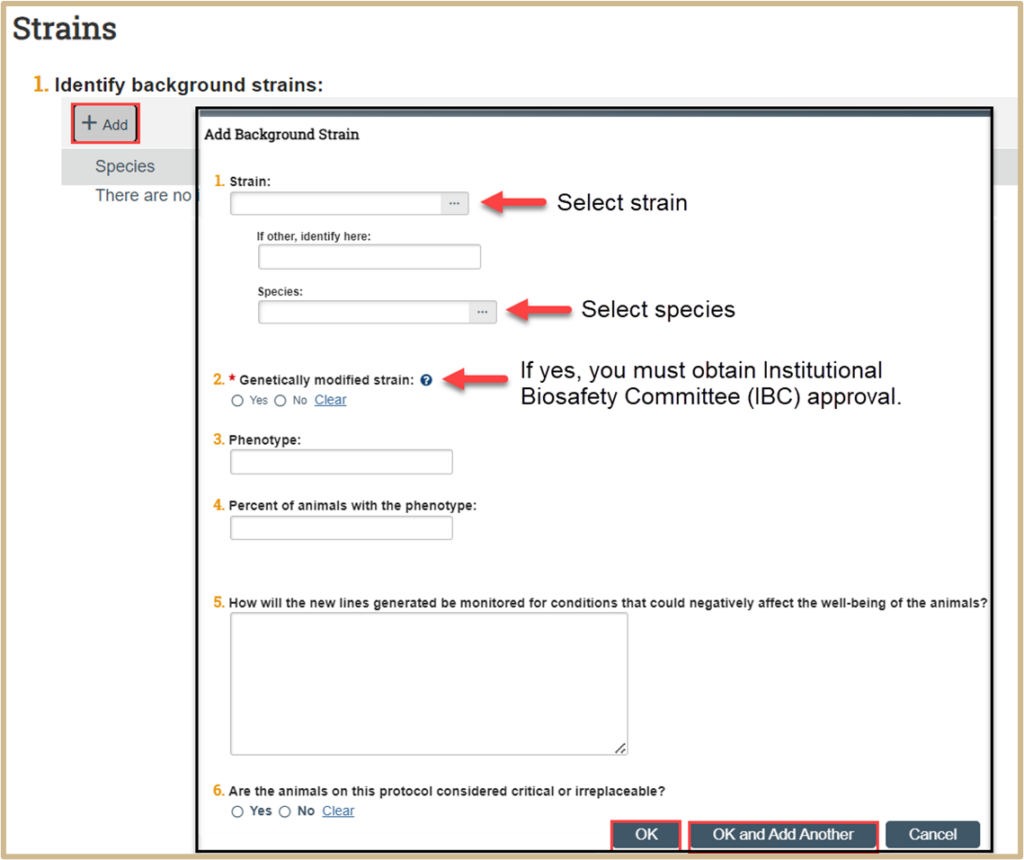
Click +Add to identify background strains.
Fill in the questions in the new popup window.
If the strain is a genetically modified strain and yes is marked in question 2, you must obtain Institutional Biosafety Committee (IBC) approval. Use this link for more information: https://www.purdue.edu/research/oevprp/regulatory-affairs/biosafety-and-rdna/
Once finished, click OK or OK and Add Another.
Select Continue to proceed to Animal Justification.
Animal Justification

![]() Use this page to explain why you require the number and types of species used in this protocol.
Use this page to explain why you require the number and types of species used in this protocol.
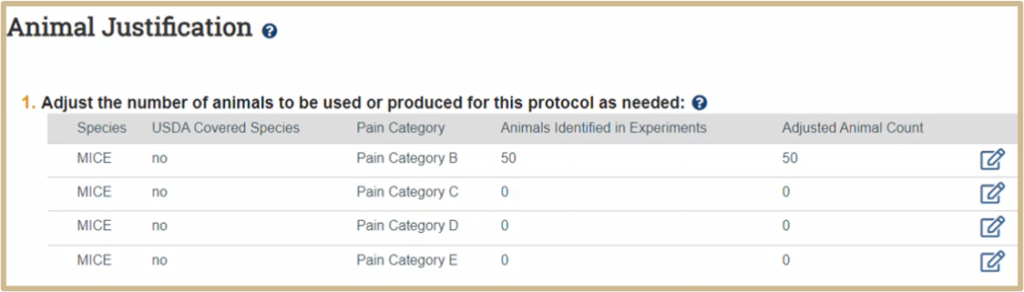
![]() NOTE: For a follow-on submission (amendment), the adjusted animal count is not automatically updated when you update the animal numbers in the experiments. You must always verify that the adjusted animal count is correct.
NOTE: For a follow-on submission (amendment), the adjusted animal count is not automatically updated when you update the animal numbers in the experiments. You must always verify that the adjusted animal count is correct.
As you justify the animal usage in this protocol, ensure you have applied the 3Rs of experimentation where possible:
- Reduce animal numbers used
- Refine animal procedures to minimize discomfort as much as possible
- Replace animals in research, teaching, and testing when possible
![]() NOTE
NOTE
The IACUC is NOT requiring you to adjust the animal count at this time while the PERA system is new. You do not have to do this. Update the adjusted animal count for each row, if:
- You will reduce the requested animal count (such as using the same animals across different experiments in this study)
- You will increase the requested animal count (such as breeding colonies within the study)
Scenario 1 – Reducing Adjusted Animal Count: Experiment A requires 60 mice and Experiment B requires 120 mice. The total number of mice in the Animals Identified in Experiments column will auto-populate to 180. In both Experiment A and Experiment B, there are 20 mice used as controls. Between the two experiments, this would equal 40 mice. However, the same mice will be used for both Experiment A and Experiment B. To accurately represent the total number of mice required for these experiments, adjust the count from 180 to 160 in the Adjusted Animal Count column.
| Species | Animals Identified in Experiments | Adjusted Animal Count |
| Mouse | 180 | 160 |
Scenario 2 – Increasing Adjusted Animal Count: Experiment C requires 50 experimental rats. However, 10 additional rats will be required for breeding that were not included in the experimental total, and therefore not represented in the Animals Identified in Experiments column. To accurately represent the total number of rats required, adjust the count from 50 to 60 in the Adjusted Animal Count column.
| Species | Animals Identified in Experiments | Adjusted Animal Count |
|---|---|---|
| Rat | 50 | 60 |
To adjust the animal counts:
- Click anywhere in the row.
- Type the new number into the Adjusted Animal Count column.
Tip: Do not click the X icon unless you want to discard your changes for the row. To collapse the edited rows, continue with the next steps. - For the next question, type an explanation for the adjusted counts.
- Click the Save button, which will collapse the rows.


![]() Pick from the following and copy and paste as many as you want into #5 above. The text below can be found in the Help button.
Pick from the following and copy and paste as many as you want into #5 above. The text below can be found in the Help button.
– Applicable anatomy, physiology, and/or behavioral processes
– Disease model similar/applicable to humans
– Precedent in the scientific literature
– Target species for agricultural/animal health research
– Teaching Activity
– Other
Also, supply 1-2 references to support the justification. If providing references is not appropriate (e.g., teaching activity), please provide a brief statement supporting the chosen species.

![]() Federal regulations mandate consideration of the principles of the 3Rs (Reduction, Refinement, and Replacement) when conducting animal use activities. Provide a summary of considerations to ensure you have applied 3Rs principles:
Federal regulations mandate consideration of the principles of the 3Rs (Reduction, Refinement, and Replacement) when conducting animal use activities. Provide a summary of considerations to ensure you have applied 3Rs principles:
- How was it determined that alternatives (e.g., less painful/distressful animal procedures, use of phylogenetically lower species or non-animal procedures) could not be substituted (i.e., why live animals must be used)?
- “Alternatives” refers to methods, models, and approaches that result in the reduction of the number of animals used, that incorporate refinements of procedures which result in the lessening of pain or distress to animals, or that provide for the replacement of animals with non-whole animal systems or the replacement of one animal species with another, particularly if the substituted species is non-mammalian or invertebrate. There must be a written narrative description of the methods and sources which were consulted to determine the availability of alternatives (reduction, refinements, replacement).
- https://www.purdue.edu/research/oevprp/regulatory-affairs/animal-research/iacuc/alternatives-three-r.php
- https://olaw.nih.gov/education/educational-resources/webinar-2021-12-09.htm
- DO NOT INCLUDE YOUR KEYWORDS AND DATABASES SEARCHED IN THIS SECTION
![]() Add any information that helps justify the numbers and/or types of species used in the protocol. These documents may elaborate on how you have applied the 3Rs of experimentation:
Add any information that helps justify the numbers and/or types of species used in the protocol. These documents may elaborate on how you have applied the 3Rs of experimentation:
- Reduce animal numbers used
- Refine animal procedures to minimize discomfort as much as possible
- Replace animals in research, teaching, and testing when possible

 Only if you selected Commercial Vendor, Direct Farm Purchase, Humane Society, Other Institution, Other Vendor, Sale Barn/Auction, or Other.
Only if you selected Commercial Vendor, Direct Farm Purchase, Humane Society, Other Institution, Other Vendor, Sale Barn/Auction, or Other.
Select Continue to proceed to Alternatives Searches and Duplication.
Alternatives Searches and Duplication
![]() Use this page to record what you have done to:
Use this page to record what you have done to:
- Ensure the same research has not already been performed.
- Find alternatives for procedures causing more than momentary pain and/or distress (category D or E).
Using alternatives is one way to apply the 3Rs of experimentation:
- Reduce animal numbers used.
- Refine animal procedures to minimize discomfort as much as possible.
- Replace animals in research, teaching, and testing when possible.
![]() NOTE: You are not required to answer Question #1, “Procedures causing pain or distress”, in the slide-in below titled “Add Procedure Search Details”.
NOTE: You are not required to answer Question #1, “Procedures causing pain or distress”, in the slide-in below titled “Add Procedure Search Details”.

![]() Record your alternative searches. You MUST search a minimum of two databases. For #5 in the slide-in above, always put in this section, “See #6 below for my keywords and search strings.”
Record your alternative searches. You MUST search a minimum of two databases. For #5 in the slide-in above, always put in this section, “See #6 below for my keywords and search strings.”
When you initiate a triennial review, your previous search records are altered to remove the search dates. The search databases and keywords are retained.
To complete the triennial review, update each search record to reflect the details of your recent searches. The date of each search must be recorded. If no longer applicable, you can delete a search record by clicking the X on the right.
Click +Add to record all searches for alternatives that causes pain or distress.
Fill in the questions in the new popup window.

Once finished, click OK or OK and Add Another.
Select Continue to proceed to Animal Housing and Use: Breeding
Animal Housing and Use

Breeding


 Only if you selected No for question #6.
Only if you selected No for question #6.

Select Continue to proceed to House and Use.
Housing and Use
In #1 above, you can select multiple housing locations.

In #3 above, you can select more than one animal use (lab, surgery) area.
 Fill out question 5 ONLY if you checked any animal use areas above where procedures and/or surgery will be performed. You MUST provide the building(s) and room number(s) according to the length of time animals will be kept in the procedure or surgery area:
Fill out question 5 ONLY if you checked any animal use areas above where procedures and/or surgery will be performed. You MUST provide the building(s) and room number(s) according to the length of time animals will be kept in the procedure or surgery area:
Disposition


 Only if Other was selected.
Only if Other was selected.

Please note that Euthanasia specific questions are embedded in the Euthanasia Procedure Form. If you are performing Euthanasia, you MUST create a Euthanasia Procedure Form for each species you plan to euthanize.
Select Continue to proceed to Custom Pages: Veterinary Care.
Custom Page

Veterinary Care

 Only if No was selected.
Only if No was selected.


![]() NOTE:
NOTE:
You must enter N/A in #3 above to proceed through the application.
Select Continue to proceed to Hazard and Occupational Health and Safety.
Hazard and Occupational Health and Safety

![]() NOTE
NOTE
It is the Principal Investigator’s responsibility to inform all personnel, including research personnel and animal care staff, of potential hazards.
All hazards (e.g., biological, chemical/drug, and radioactive) must be properly identified and handled according to EHS (formerly REM) policy/procedures, the Animal Safety Verification Form for the protocol, and/or CMAF training.
Contact Environmental Health & Safety (765-494-6371) if you have any questions concerning the completion of this section. Please respond to all that apply below:


 Only if No is selected on #6.
Only if No is selected on #6.

Select Continue to proceed to Supporting Documents.
Supporting Documents
![]() Add any information that you did not include on other pages, for example:
Add any information that you did not include on other pages, for example:
- Flowcharts outlining the flow of research or proposed experiments
- Detailed explanations of science, including scientific notations or graphics
- Clinical trials documents related to the submission
- Other information relevant to the protocol that is not attached elsewhere (e.g. breeding or restraint device details, safety information, or departures from the Guide)
These documents will list on the Documents tab of the protocol workspace.
Click Finish when done.
Content Updated:



