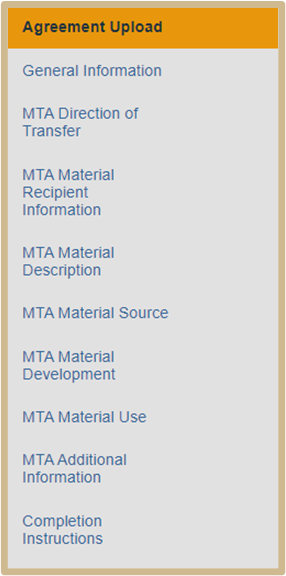
MTA Sending Materials
Process Overview
This guide shows how to create and fill out a Material Transfer Agreement (MTA) – Sending Materials SmartForm request.
Roles
SPS requires the faculty member to certify the MTA submission. Contracting cannot begin review until they have seen certification by the faculty member. If someone submits the MTA SmartForm other than the PI, SPS Contracting will need to reach out to the PI to obtain their certification that the information submitted is accurate.
Content
Scroll down to receive the procedure step-by-step or select a step below.

How to Create a Material Transfer Agreement – Sending Materials
To create an MTA, you will first create an agreement and specify that you will need an MTA on the Agreement Upload SmartForm page. You will also need to complete all the required fields on the General Information SmartForm page.
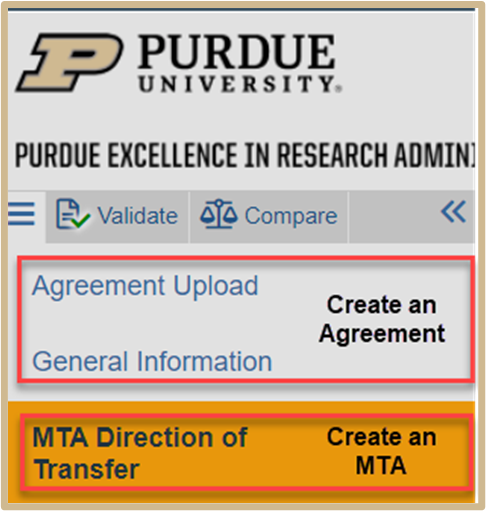
1. Direction of Transfer
When the MTA agreement type option is selected, the MTA Direction of Transfer page will appear. You will need to indicate whether you are receiving or sending materials. The subsequent SmartForms and questions will change depending on which answer is chosen.
1.
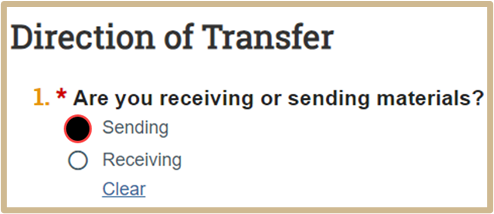
2.
2. Sending Materials
You will fill in the following pages and questions to complete the MTA request when Purdue is selected as the sender of the materials. Additional pages may appear depending on selections made during the completion of the SmartForm.
MTA Material Recipient Information
Questions are numbered below for the purpose of following the process in order. Question numbers in PERA may change based on responses.
1.
 The MTA Export Control SmartForm will appear if yes is selected.
The MTA Export Control SmartForm will appear if yes is selected.

2.
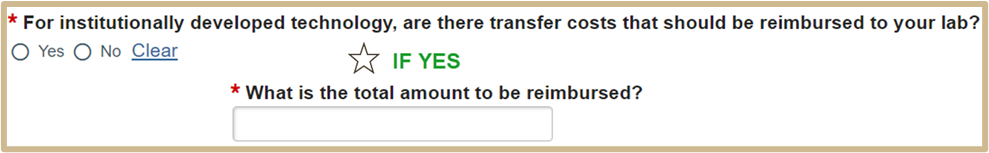
![]() NOTE
NOTE
Reimbursement would include shipping costs, handling costs, but not payment for the material. The conditional field must be a number or dollar amount.
3.

4.
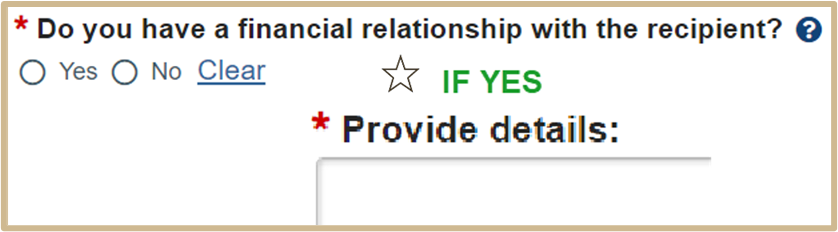
![]() You have a financial relationship with the recipient if you have or your immediate family has received anything of economic value from the recipient, such as:
You have a financial relationship with the recipient if you have or your immediate family has received anything of economic value from the recipient, such as:
- Salary or payment for services (e.g., consulting)
- Equity interest (e.g., stocks, stock options, other ownership interest)
- Income-related to intellectual property (e.g., patents, copyrights)
- Reimbursed travel expenses
- Gifts, memberships, honoraria, etc.
5.
6.
Select Continue to proceed to MTA Material Description OR MTA Export Control.
2. MTA Export Control

This page will appear as a smart form page to complete if you indicated you are exporting materials outside of the United States. Additional information may be required depending on your answer selections.
![]() NOTE
NOTE
If you have questions about your answers, please contact the Research Security and Export Control Office. Sponsored Programs Services is not able to provide guidance on export control matters.
1.

2.

3.

4.
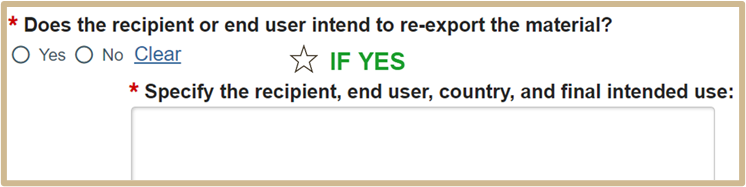
5.

(1) The term “biological agent” means any microorganism (including, but not limited to, bacteria, viruses, fungi, rickettsiae or protozoa), or infectious substance, or any naturally occurring, bioengineered or synthesized component of any such microorganism or infectious substance, capable of causing—
- death, disease, or other biological malfunction in a human, an animal, a plant, or another living organism;
- deterioration of food, water, equipment, supplies, or material of any kind; or
- deleterious alteration of the environment;
(2) the term “toxin” means the toxic material or product of plants, animals, microorganisms (including, but not limited to, bacteria, viruses, fungi, rickettsiae or protozoa), or infectious substances, or a recombinant or synthesized molecule, whatever their origin and method of production, and includes—
- any poisonous substance or biological product that may be engineered as a result of biotechnology produced by a living organism; or
- any poisonous isomer or biological product, homolog, or derivative of such a substance;
(3) the term “delivery system” means—
- any apparatus, equipment, device, or means of delivery specifically designed to deliver or disseminate a biological agent, toxin, or vector; or
- any vector;
(4) the term “vector” means a living organism, or molecule, including a recombinant or synthesized molecule, capable of carrying a biological agent or toxin to a host; and
(5) the term “national of the United States” has the meaning prescribed in section 101(a)(22) of the Immigration and Nationality Act (8 U.S.C. 1101(a)(22)).
6.
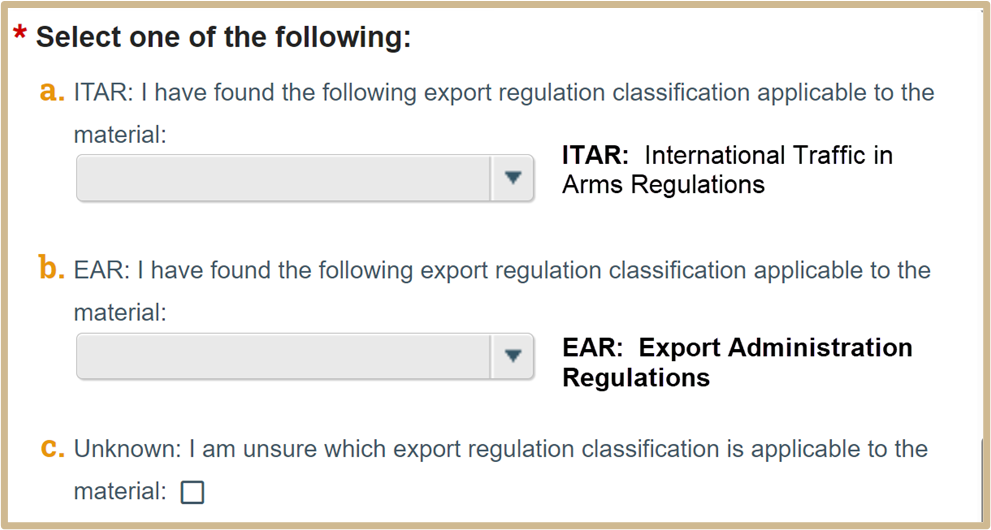
Select Continue to proceed to MTA Material Description.
3. MTA Material Description

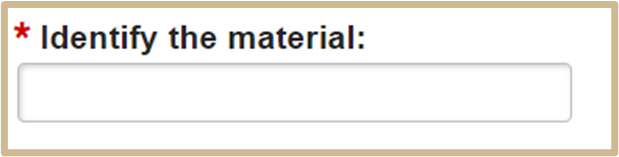
1.
2.
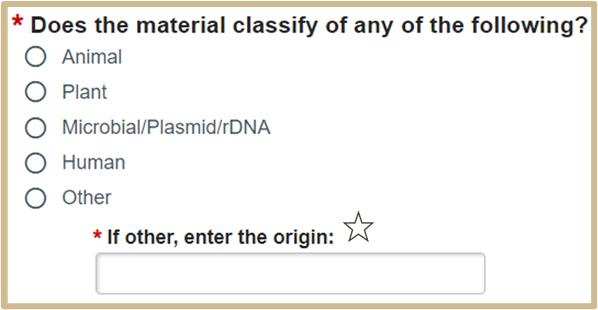
![]() If you are transferring multiple materials select “other” and use the MTA Additional Information SmartForm page to provide a detailed description of each material. If any of the materials classify as human, please select human for this question. Additional SmartForm pages will populate when “human” is selected.
If you are transferring multiple materials select “other” and use the MTA Additional Information SmartForm page to provide a detailed description of each material. If any of the materials classify as human, please select human for this question. Additional SmartForm pages will populate when “human” is selected.
![]() NOTE
NOTE
If there is more than one material in this MTA and neither classify as Human, please select other and enter both materials in the text. If there is more than one material in this MTA and any classify as Human, please select Human here so that additional questions will populate that are needed to process this request.
3.
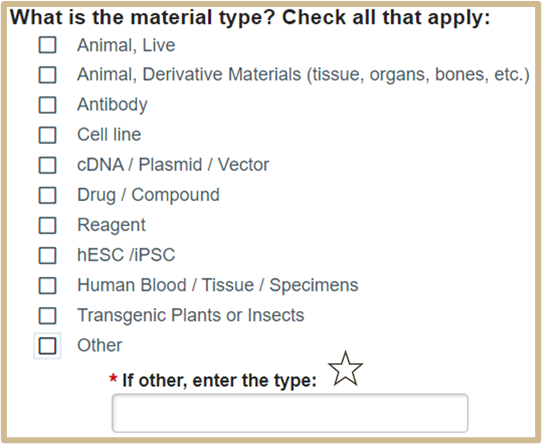
4.
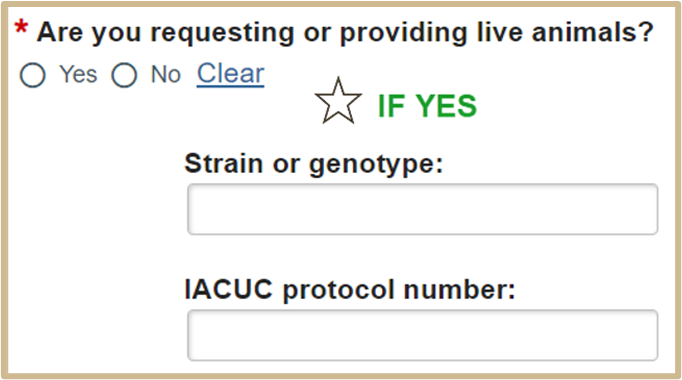
The Strain or Genotype and IACUC protocol number will only populate if you are requesting live animals
5.
6.
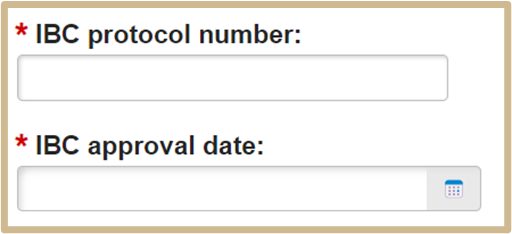
The IBC protocol number and IBC approval date will only populate if your material type requires this information.

Material Types that require IBC protocol numbers and approval dates: animal, derivative materials (tissue, organs, bones, etc.), antibody, cell line, cDNA/plasmid/vector, drug/compound, hESC/iPSC, human blood/tissue/specimens, transgenic plants or insects
![]() NOTE
NOTE
If you checked more than one material type, you will need to provide all protocol numbers and their respective dates. Use the MTA Additional Information form at the end of this SmartForm to provide additional protocol number and dates.
7.
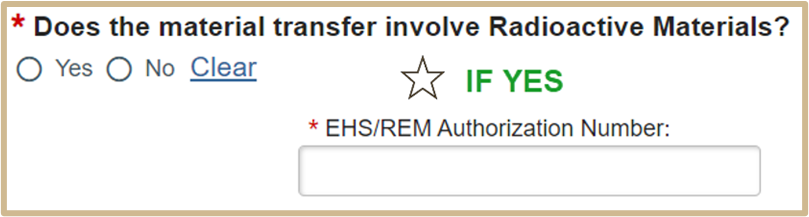
Select Continue to proceed to Human Material Description only if Human was selected in question #2 or MTA Material Source if any other material was chosen.
 Complete only if Human was selected in question #2.
Complete only if Human was selected in question #2.
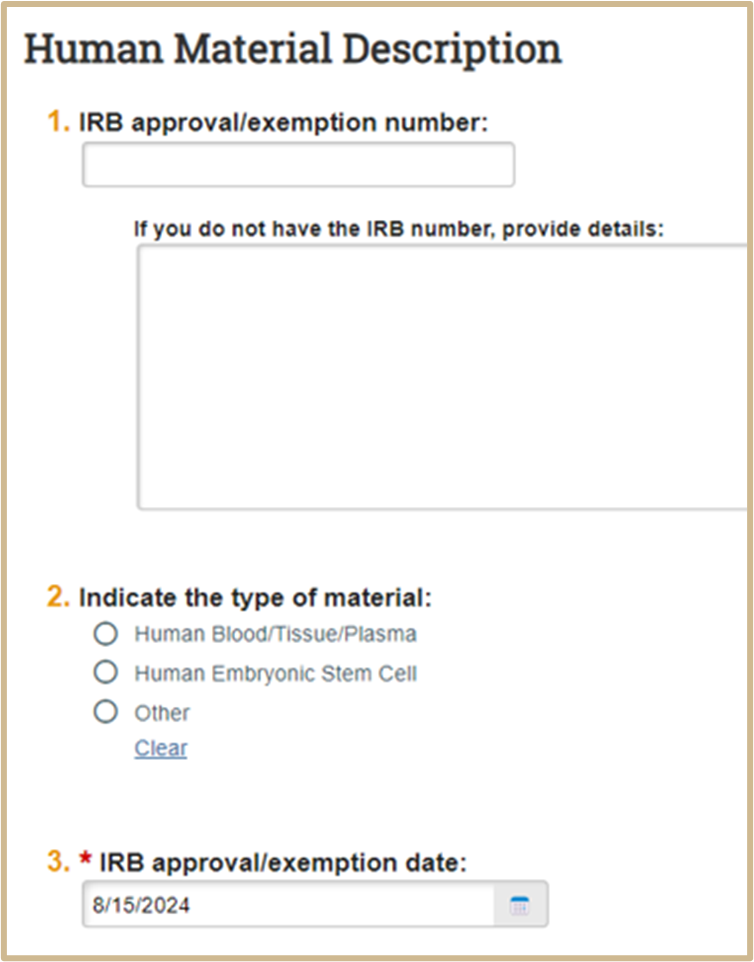
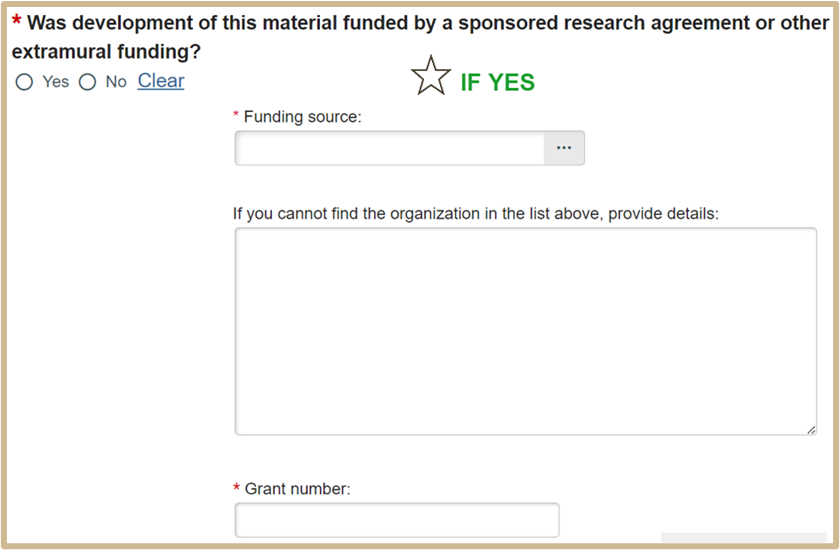
4. MTA Material Source

Questions 1 and 2 may be left blank if developed exclusively at Purdue.
1.
2
3.

4.
5.
Select Continue to proceed to MTA Material Development.
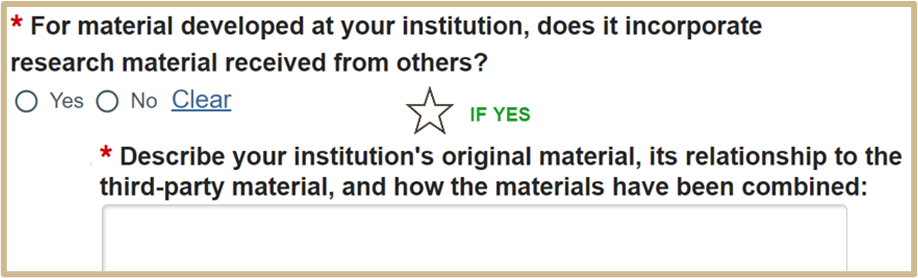
5. MTA Material Development

1.
1.

Select Continue to proceed to MTA Third-Party Material OR MTA Material Use.
6. MTA Third-Party Material
This page will appear as a smart form page to complete if you indicated the material developed at Purdue incorporates materials received from others. Additional information may be required depending on your answer selections.

Provide the information of the source organization of the material, if it did not originate at Purdue.
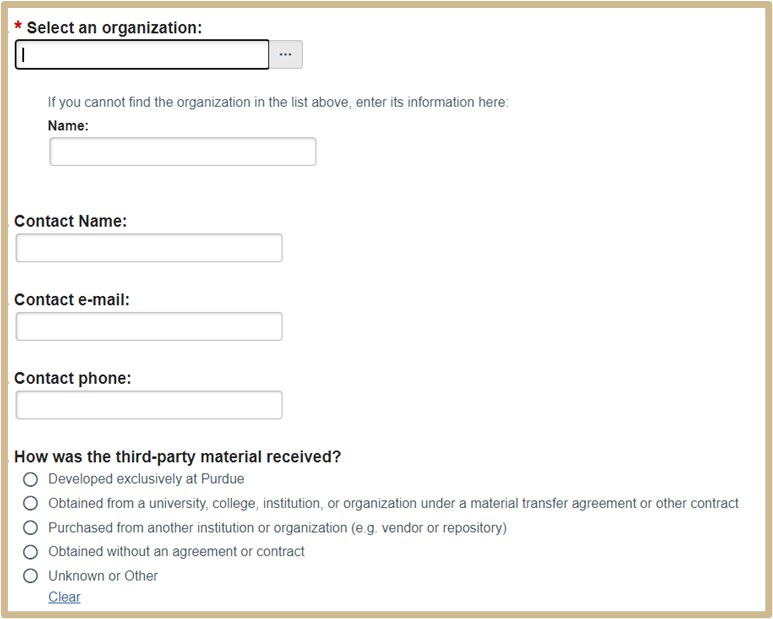
Once complete, select Continue to proceed to Material Use.
7. MTA Material Use

1.

2.

3.
![]() This field must contain a number or dollar amount. That amount may be 0. If unknown, use your best estimate, or use 0.00 and provide comments below (such as “Unsure – Material is not commercially available”).
This field must contain a number or dollar amount. That amount may be 0. If unknown, use your best estimate, or use 0.00 and provide comments below (such as “Unsure – Material is not commercially available”).
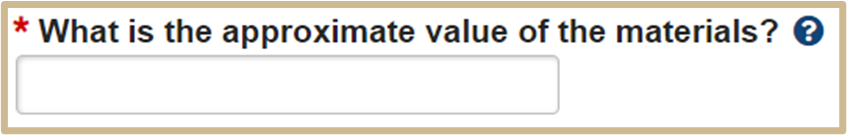
4.

5.

6.
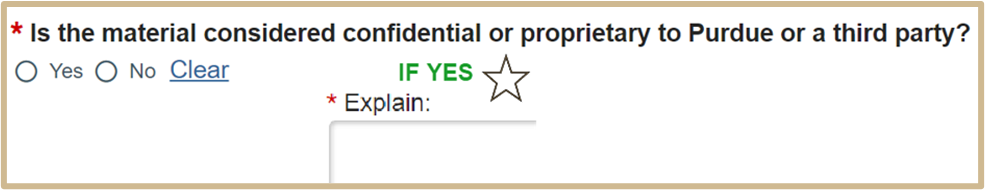
Select Continue to proceed to MTA Additional Information.
8. MTA Additional Information

1.
Select Continue to proceed to Completion Instructions.
9. Completion Instructions

The Completion Instructions page will be shown last.
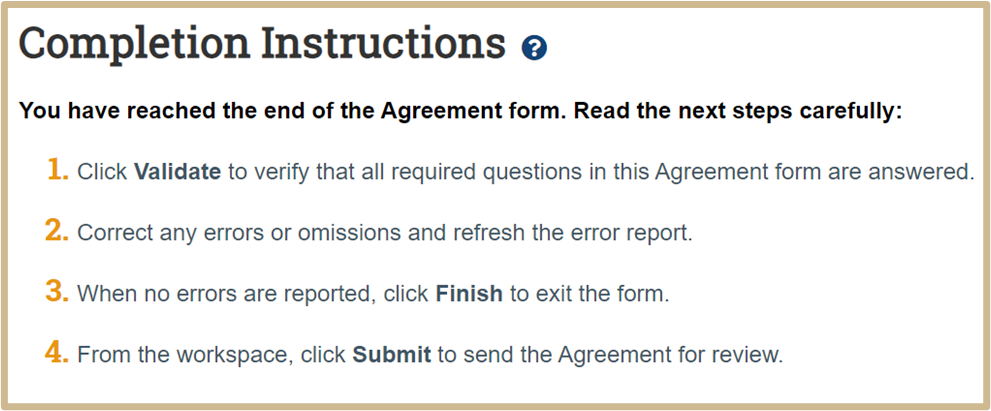
![]() When you click Finish on the Completion Instructions page, you have not yet finished all the steps to send the agreement for review. You have the option to continue editing the agreement. Before the Agreement Office can review it, you must submit it for review from the workspace. You will find the submit options after you click Finish.
When you click Finish on the Completion Instructions page, you have not yet finished all the steps to send the agreement for review. You have the option to continue editing the agreement. Before the Agreement Office can review it, you must submit it for review from the workspace. You will find the submit options after you click Finish.
Your request is not complete and will not be processed until it is submitted.
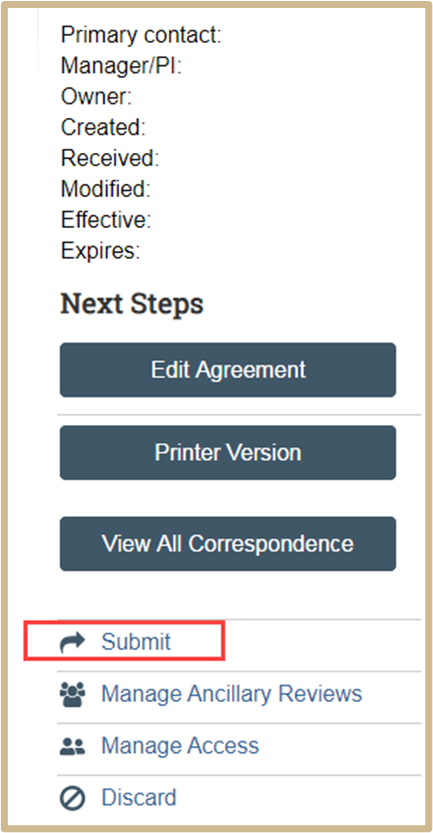
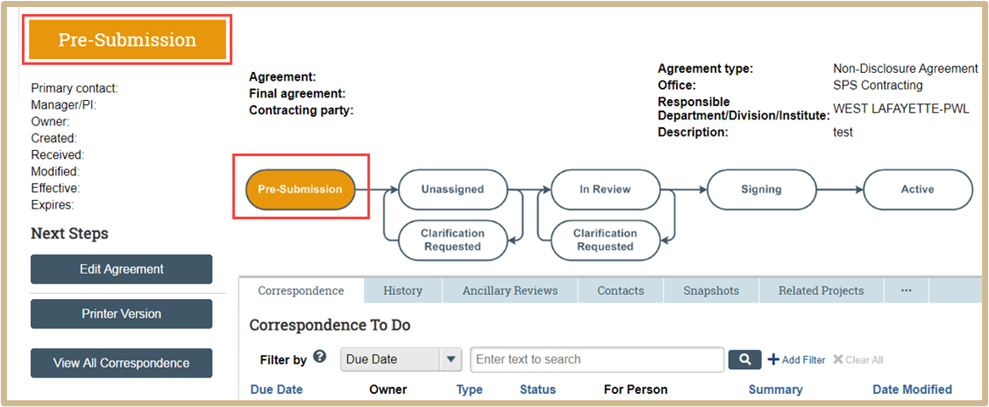
Your project has not been properly submitted if the flow chart has Pre-Submission indicated.
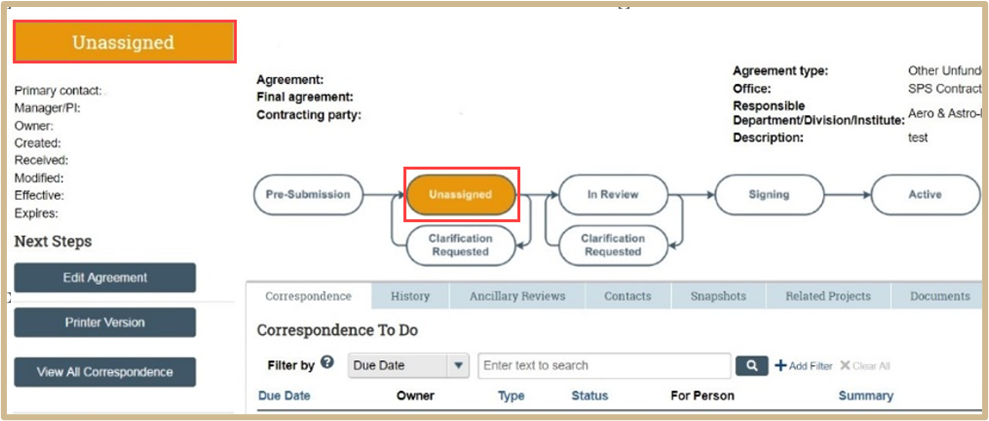
Unassigned will be highlighted when properly submitted.
Content Updated: